Biochemical diagnosis of metachromatic leukodystrophy (MLD) is usually performed by measuring arylsulfatase A (ARSA; EC3.1.6.8) activity with artificial substrates (p-nitrocatechol sulfate or 4-methylumbelliferyl sulfate) in leukocytes or cultured skin fibroblasts [1, 2]. Unfortunately, these artificial substrates are also substrates for several other enzymes, including arylsulfatase B [3, 4]. Thus, quantitation of residual activity could be inaccurate, especially in the context of MLD variants and ARSA pseudodeficiency. In this study, we evaluated the feasibility of ultra-performance liquid chromatography (UPLC)-tandem mass spectrometry (MS/MS) in a leukocyte ARSA assay using a natural sulfatide substrate, and we compared this approach with a traditional spectrophotometric assay using a synthetic substrate.
The substrate and internal standard (IS) were N-octadecanoyl-sulfatide and N-octadecanoyl-D35-psychosine (Matreya LLC, Pleasant Gap, PA, USA), respectively. We used C18 β-D-glucosyl ceramide (Avanti Polar Lipids Inc., Alabaster, AL, USA) instead of C18 β-D-galactosyl ceramide as the standard because of commercial availability. The enzyme reaction cocktail contained 2.08 g/L sodium taurodeoxycholate, 33 mmol/L MnCl2, 0.08 mol/L sodium acetate buffer (pH 4.5), and 6.20 µmol/L substrate. Seventy microliters of reaction cocktail and 30 µL of sonicated leukocyte solution were incubated at 37℃ for 1 hr and were quenched with 100 µL of ethyl acetate-methanol solution. Ten microliters of 1 µmol/L IS solution and 300 µL of both ethyl acetate and water were added to the reaction vial and centrifuged at 15,700 g for 10 min. Two hundred microliters of the top organic layer was dried under N2 gas and resuspended in 70 µL of methanol. And 7 µL was injected into a Waters ACQUITY UPLC system (Waters, Milford, MA, USA). The mobile phase was a mixture that was 97% methanol containing 3% of 0.05 mol/L ammonium formate solution in water at a flow rate of 0.5 mL/min. A Quattro Premier XE MS/MS (Waters) was operated by using the following settings: cone voltage, 25, 30, and 25 V; collision energy, 35, 40, and 35 V; and multiple reaction monitoring transition in positive ion mode, m/z 808.5→264.3, m/z 763.7→265.3, and m/z 728.5→264.3 for the substrate, IS, and product, respectively. The amount of product was calculated from the calibration curves constructed with five concentrations of C18 ß-D-glucosyl ceramide (0-687 nmol/L), and the enzyme activities were expressed in nmol/min/mg protein.
Substrates, products, and IS were fully separated by using UPLC with an HSS T3 1.8-µm column (2.1×50 mm) with a 3 min chromatographic separation time (Fig. 1). The amount of product obtained was proportional to the volume of leukocytes used in the assay (10, 20, 30, 40, and 50 µL of leukocyte extract) and increased linearly with the incubation period (0, 0.5, 1, 2, and 3 hr). We chose a 30 µL leukocyte volume and a 1 hr incubation time for all subsequent enzymatic assays. The within- and between-run imprecision (CVs), as determined by 10 replicated analyses and 5 consecutive runs using a normal control sample, were 7.7% and 14.5%, respectively.
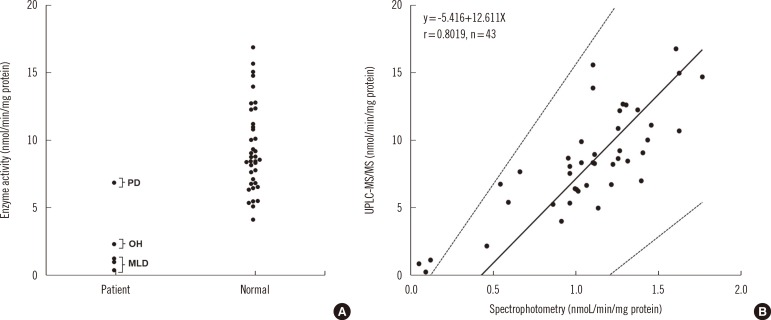
To validate the capability of our system to detect MLD patients, three MLD patients, one pseudodeficiency, one obligate heterozygote and 38 normal adults were examined. All patients exhibited reduced enzyme activity using synthetic substrates, confirmed by mutation analysis. As expected, the leukocytes of MLD patients exhibited consistently lower enzyme activities than those of the obligate heterozygote, pseudodeficiency, and normal adults without overlapping values (Fig. 2A). The provisional cut-off value was estimated as 1.23 mmol/min/mg protein. Passing-Bablok regression analysis revealed that the UPLC-MS/MS method and the traditional colorimetric assay [1] compared favorably (r=0.8019) (Fig. 2B).
Recently, reports on multiplex enzyme assay screening of dried blood spots (DBS) for lysosomal storage disorders have engendered interest in the use of MS/MS for newborn screening [5, 6]. Although assays using natural sulfatide substrates are more complicated because of the poor water solubility of sulfatides [7], utilization of natural substrates is increasingly required becausethe ARSA assay may soon be incorporated into newborn screening programs. In this regard, mass spectrometry is accepted as a valuable tool for the analysis of lipids and lipid-metabolizing enzymes using a natural substrate system. In this study, we investigated the feasibility of UPLC-MS/MS for use in a leukocyte ARSA assay using a natural sulfatide substrate. To the best of our knowledge, this is the first study to report the feasibility of UPLC-MS/MS for diagnosing MLD. The assay performance for our devised method in terms of precision and correlation with a traditional spectrophotometric method was within the generally acceptable standard and could be adopted for newborn screening of DBSs for MLD. More experiments would be needed to develop the assay for routine application.
Acknowledgments
This study was supported by a grant from the Korea Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A120030).
References
1. Baum H, Dodgson KS, Spencer B. The assay of arylsulphatases A and B in human urine. Clin Chim Acta. 1959; 4:453–455. PMID: 13663253.

2. Harinath BC, Robins E. Arylsulphatases in human brain: assay, some properties, and distribution. J Neurochem. 1971; 18:237–244. PMID: 5550089.

3. Lee-Vaupel M, Conzelmann E. A simple chromogenic assay for arylsulfatase A. Clin Chim Acta. 1987; 164:171–180. PMID: 2885112.

4. Rip JW, Gordon BA. A simple spectrophotometric enzyme assay with absolute specificity for arylsulfatase A. Clin Biochem. 1998; 31:29–31. PMID: 9559221.

5. Spacil Z, Tatipaka H, Barcenas M, Scott CR, Turecek F, Gelb MH. High-throughput assay of 9 lysosomal enzymes for newborn screening. Clin Chem. 2013; 59:502–511. PMID: 23315484.

6. Han M, Jun SH, Song SH, Park KU, Kim JQ, Song J. Use of tandem mass spectrometry for newborn screening of 6 lysosomal storage disorders in a Korean population. Korean J Lab Med. 2011; 31:250–256. PMID: 22016678.

7. Norris AJ, Whitelegge JP, Yaghoubian A, Alattia JR, Privé GG, Toyokuni T, et al. A novel mass spectrometric assay for the cerebroside sulfate activator protein (saposin B) and arylsulfatase A. J Lipid Res. 2005; 46:2254–2264. PMID: 16061947.

Fig. 1
Representative ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) multiple reaction monitoring (MRM) chromatograms from the mixture of S, IS and standard (A) and the mixture of ARSA enzyme reactions in the leukocyte samples from normal subjects (B) and metachromatic leukodystrophy patients (C).
Abbreviations: ARSA, arylsulfatase A; IS, internal standard; S, substrate; P, product.
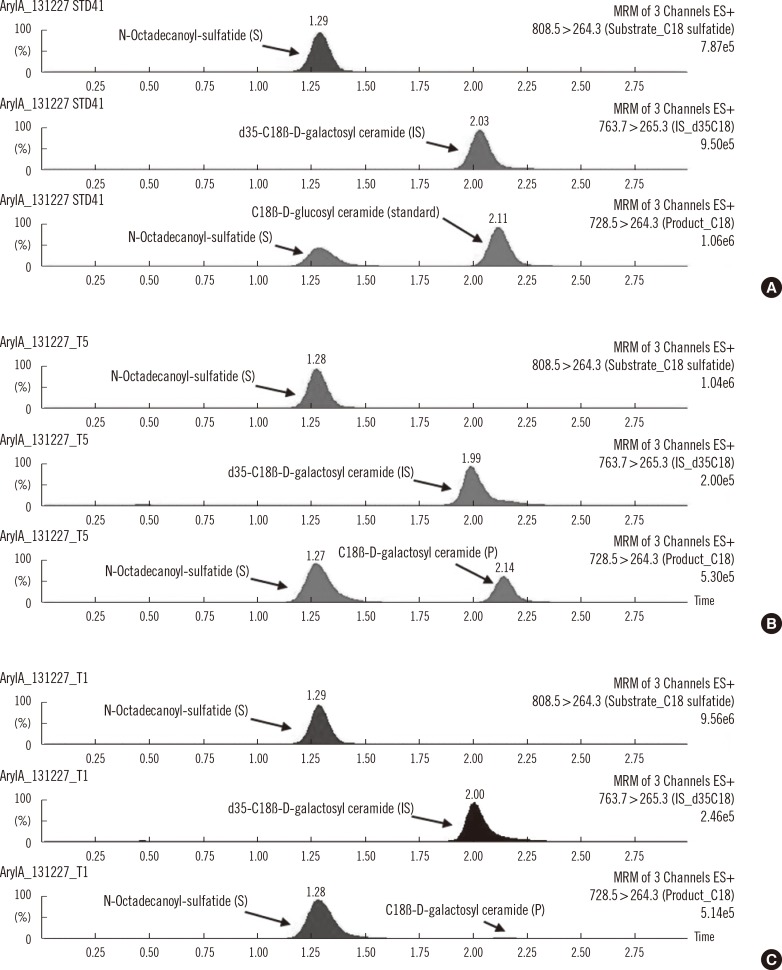
Fig. 2
Comparison of arylsufatase A activities measured by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) in leukocytes samples from three patients with MLD (all 2-yr-old females) and one patient with pseudodeficiency (PD, a 47-yr-old female), one obligate heterozygote (OH, a 33-yr-old male) and 38 normal adults (females, n=21, age=61±14 yr; males, n=17, age=57±17 yr) (A). Passing-Bablok regression analysis between the UPLC-MS/MS method and the spectrophotometric assay (B).
Abbreviations: PD, pseudodeficiency; OH, obligate heterozygote; MLD, metachromatic leukodystrophy.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download