Hemophagocytic lymphohistiocytosis (HLH) can be divided into primary (genetic or familial) and secondary (acquired or reactive) forms. Abnormal immunophenotyping and downregulation of CD5 or CD7 in T cells have been well characterized in Epstein-Barr virus (EBV)-associated HLH [1, 2]. A few studies have also reported abnormal immunophenotyping of T cells in patients with familial HLH (FHL) [1, 3]. Here, we report a case of FHL with immunophenotypically abnormal T cells and monoclonal T cells confirmed by a T-cell receptor (TCR) gene rearrangement test.
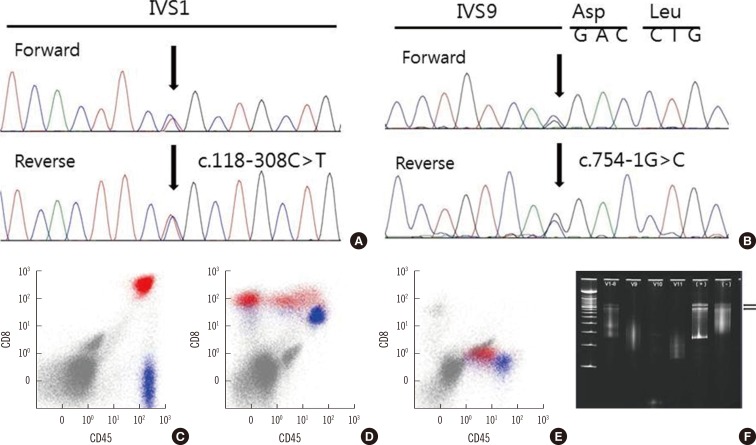
A 2-month-old infant was referred to our hospital for further evaluation and treatment of persistent fever and thrombocytopenia. Hepatosplenomegaly and petechiae were observed on physical examination. The total leukocyte count was 1.64×109/L, consisting of 65% lymphocytes, with 1% atypical lymphoid cells. The Hb level was 9.1 g/dL, and the platelet count was 20×109/L. Blood chemistry results were as follows: 570 IU/L AST, 454 IU/L ALT, 657 IU/L lactate dehydrogenase (LDH), 11.4 mg/dL total bilirubin, and 147 mg/dL fibrinogen. The prothrombin time (PT) and activated partial thromboplastin time (aPTT) was 15.3 sec (reference range; 12.6-14.9 sec) and 40.3 sec (reference range; 29.1-41.9 sec), respectively. A serological test to detect any underlying viral infection showed (+, positive; -, negative): anti-CMV IgG (+), anti-CMV IgM (-), anti-EB-VCA IgG (+), anti-EB-VCA IgM (-), EBV-EA (-), and anti-EB-NA IgG (+). The results of a chromosome study were normal (46,XY). Atypical lymphocytes were observed on peripheral blood smear (Fig. 1A). Hemophagocytic histiocytes were observed on bone marrow biopsy (Fig. 1B, C, and D). EBV was not detected by in situ hybridization. We performed mutation analysis of the FHL-related genes UNC13D and PRF1 using DNA isolated from peripheral blood. Sequencing analysis revealed compound heterozygous mutations in UNC13D [c.118-308 C>T (;)754-1G>C] (Fig. 2A and B). These mutations are frequently observed in Korean FHL patients [4]. Immunophenotyping using bone marrow aspirate revealed an abnormal population of CD8+ T cells with downregulated levels of CD5 or CD7 (14.61% of total events; Fig. 2C, D, and E). A TCR gene rearrangement study using paraffin-embedded tissue revealed T cell monoclonality [5] (Fig. 2F). The patient was treated with HLH-2004 chemotherapy and allogenic bone marrow transplantation. Bone marrow studies were performed before and after allogenic bone marrow transplantation. Hemophagocytic histiocytes were not observed in the follow-up bone marrow study. The patient remained in remission (follow-up time: 12 months after diagnosis). A familial study for UNC13D gene mutation was not performed.
Abnormal immunophenotype of CD8+ T cells with downregulation of CD5 is a common finding in HLH associated with viruses, especially EBV-associated HLH [1, 6, 7]. McCall et al. [1] reported that 6 of 9 cases (76%) of EBV-associated HLH had expansion of CD8+ T cell populations with variable downregulation of CD5, CD7, and/or CD3, whereas 1 of 8 cases (13%) of non-EBV-associated HLH showed downregulation of CD7 expression [1]. There are few reports on abnormal immunophenotype of T cells in FHL [1, 3, 8]. Karandikar et al. [3] reported a case of FHL associated with a perforin gene mutation that showed CD8+ T cells with loss of CD5 expression. Wada et al. [8] also reported a case of FHL associated with a perforin gene mutation that showed CD8+ T cells with loss of CD5 expression. Downregulation of CD5, CD7, CD3 and aberrant expression of HLA-DR is a common finding in both FHL and EBV-associated HLH or other acquired HLH [1, 3]. In this case, downregulation of CD5 and CD7 was found, as reported previously [1, 3]. The cause of downregulation of CD5 expression in acquired HLH or FHL remains unclear. Some authors have suggested a link between CD5 downregulation in T cells and monoclonal proliferation [6].
Our case also showed monoclonality in the TCR gene rearrangement study. Monoclonality in the TCR gene rearrangement study is also a common finding in EBV-associated HLH [9]. Monoclonal T cell proliferation in FHL has also been reported [10]. Moreover, the study also reported clonal changes in T cell populations after treatment (monoclonal to polyclonal) [10]. These results might help shed light on the pathogenesis underlying clonal proliferation of T cells and downregulation of CD5 in HLH.
To the best of our knowledge, this is the first report on FHL with an abnormal immunophenotype and T cell monoclonality in Korea. Further studies are needed to characterize the immunophenotypic features and clonality of T cells in FHL and their diagnostic or clinical significance.
References
1. McCall CM, Mudali S, Arceci RJ, Small D, Fuller S, Gocke CD, et al. Flow cytometric findings in hemophagocytic lymphohistiocytosis. Am J Clin Pathol. 2012; 137:786–794. PMID: 22523218.

2. Ahn JS, Rew SY, Shin MG, Kim HR, Yang DH, Cho D, et al. Clinical significance of clonality and Epstein-Barr virus infection in adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. 2010; 85:719–722. PMID: 20652965.

3. Karandikar NJ, Kroft SH, Yegappan S, Rogers BB, Aquino VM, Lee KM, et al. Unusual immunophenotype of CD8+ T cells in familial hemophagocytic lymphohistiocytosis. Blood. 2004; 104:2007–2009. PMID: 15205266.

4. Seo JY, Song JS, Lee KO, Won HH, Kim JW, Kim SH, et al. Founder effects in two predominant intronic mutations of UNC13D, c.118-308C>T and c.754-1G>C underlie the unusual predominance of type 3 familial hemophagocytic lymphohistiocytosis (FHL3) in Korea. Ann Hematol. 2013; 92:357–364. PMID: 23180437.
5. Signoretti S, Murphy M, Cangi MG, Puddu P, Kadin ME, Loda M. Detection of clonal T-cell receptor gamma gene rearrangements in paraffin-embedded tissue by polymerase chain reaction and nonradioactive single-strand conformational polymorphism analysis. Am J Pathol. 1999; 154:67–75. PMID: 9916920.
6. Toga A, Wada T, Sakakibara Y, Mase S, Araki R, Tone Y, et al. Clinical significance of cloned expansion and CD5 down-regulation in Epstein-Barr Virus (EBV)-infected CD8+ T lymphocytes in EBV-associated hemophagocytic lymphohistiocytosis. J Infect Dis. 2010; 201:1923–1932. PMID: 20443735.
7. Lin MT, Chang HM, Huang CJ, Chen WL, Lin CY, Chuang SS. Massive expansion of EBV+ monoclonal T cells with CD5 down regulation in EBV-associated haemophagocytic lymphohistiocytosis. J Clin Pathol. 2007; 60:101–103. PMID: 17213357.

8. Wada T, Sakakibara Y, Nishimura R, Toma T, Ueno Y, Horita S, et al. Down-regulation of CD5 expression on activated CD8+ T cells in familial hemophagocytic lymphohistiocytosis with perforin gene mutations. Hum Immunol. 2013; 74:1579–1585. PMID: 24051121.

9. Imashuku S, Hibi S, Tabata Y, Itoh E, Hashida T, Tsunamoto K, et al. Outcome of clonal hemophagocytic lymphohistiocytosis: analysis of 32 cases. Leuk Lymphoma. 2000; 37:577–584. PMID: 11042518.

10. Ishii E, Kimura N, Kato K, Sako M, Nagano M, Nakagawa A, et al. Clonal change of infiltrating T-cells in children with familial hemophagocytic lymphohistiocytosis: possible association with Epstein-Barr virus infection. Cancer. 1999; 85:1636–1643. PMID: 10193957.
Fig. 1
Peripheral blood smear, bone marrow biopsy and aspirate. (A) Pancytopenia and atypical lymphoid cells were observed in peripheral
blood smear (Wright-Giemsa stain; magnification, ×1,000). (B) Hemophagocytic histiocytes were frequently observed in bone marrow aspiration (Wright-Giemsa stain; magnification, ×1,000). (C) Histiocytes were high in number in the biopsy section (Hematoxylin-Eosin stain; magnification ×200). (D) CD68 immunization (magnification, ×400).
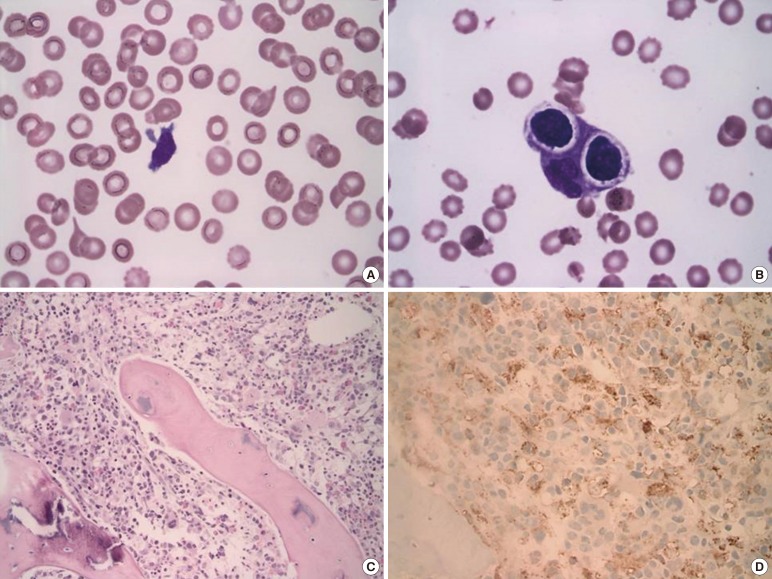
Fig. 2
Sequencing analysis, flow cytometry results, and single-strand conformational polymorphism (SSCP) analysis of the T cell receptor by using paraffin-embedded tissue samples. (A) Compound heterozygous mutations in the UNC13D gene were observed: C to T substitution at nucleotide -308 of intron 1, relative to the cDNA positioned between nucleotides 117 and 118 (118-308C>T). (B) G to C substitution at nucleotide -1 of intron 9, relative to the cDNA positioned between nucleotides 753 and 754 (754-1G>C). The CD3+/CD8+ T cells (red). (C) showed variable downregulation of CD5 or CD7 (lack of expression) compared with CD3+/CD4+ cells (blue, presumably normal T cells; D and E). (F) DNA was extracted from paraffin-embedded bone marrow tissue. Consensus primer for Vγ1-8, Vγ9-11 was used for amplification. PCR products were analyzed by SSCP, which separated DNA fragments according to nucleotide sequence. Well-defined, distinct bands were considered evidence of monoclonality. The two black arrows indicate the distinct monoclonal band at the level of the lowest smear in the polyclonal control (-). Monoclonality was observed in Vγ1-8, and polyclonality was observed in Vγ9-11.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download