A 73-yr-old man was evaluated for fever and multiple palpable nodules in his neck and inguinal areas. Biopsy of a cervical lymph node demonstrated polymorphic infiltrates including T-cells with abundant clear cytoplasm (clear cells), follicular dendritic cells, and plasma cells in the background of prominent arborizing high endothelial venules. The involved lymph node had typical histological and immunohistochemical properties characteristic of angioimmunoblastic T-cell lymphoma (AITL), except for the absence of CD10 expression. Lymphoplasmacytic lymphoma was excluded. A staging computed tomographic scan showed generalized lymphadenopathy in his chest, abdomen, and pelvis, as well as hepatosplenomegaly.
Laboratory findings demonstrated the presence of leukocytosis (30.5×109/L). A peripheral blood (PB) smear revealed rouleaux formations and numerous circulating plasma cells, with plasmacytoid lymphocytes accounting for 25% of all nucleated cells. Protein electrophoresis and immunofixation studies of serum indicated IgMκ monoclonal gammopathy (IgM level, 53.8 g/L; normal range, 0.4-2.3 g/L). A bone marrow (BM) biopsy revealed 80% hypercellularity with focal clusters of CD3+ malignant T-cells and diffuse CD138+ clonal plasma cells at proportions of 10% and 30%, respectively (Fig. 1). The CD3+ T-cells were medium-sized lymphoid cells with moderate amounts of clear cytoplasm and slightly indented nuclei with coarse and condensed chromatin. In concordance with the monoclonal gammopathy found in the serum, immunohistochemical analysis of mixed plasma cells revealed a pattern restricted to κ light chain reactivity. Rare scattered cells were found positive for Epstein-Barr virus (EBV) by using in situ hybridization. Analysis for the expression of CD20 indicated relatively few mature B-cells.
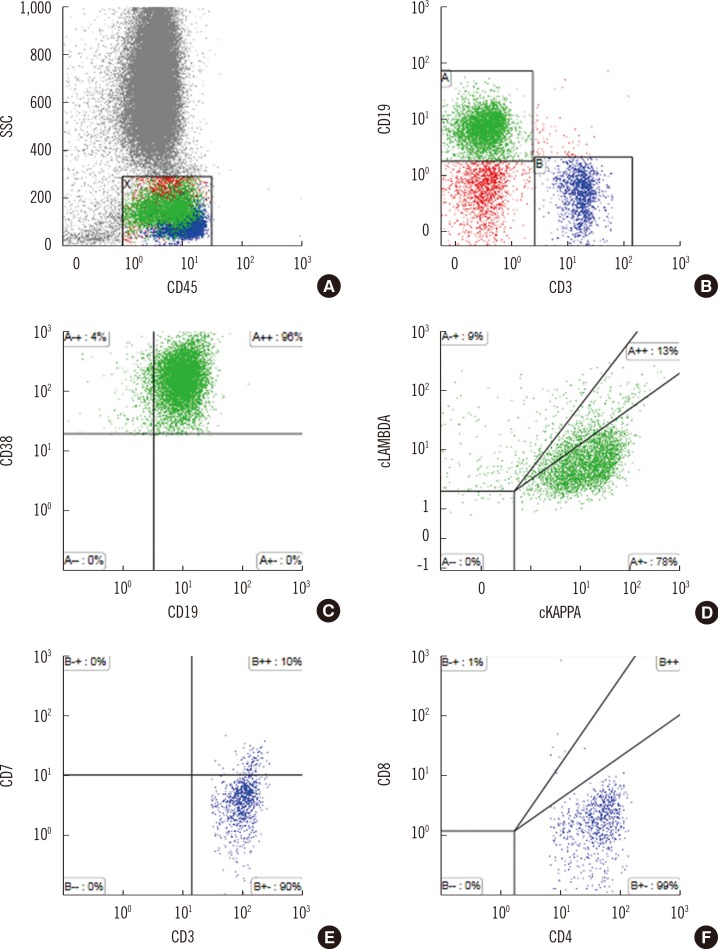
Flow cytometry analysis of circulating lymphocytes revealed a B-cell population expressing CD19, but the cells lost CD20 without surface Ig expression (25.12% of total events). Instead, these cells intensely expressed CD38 with a fraction of the cells also expressing CD138. Almost all CD38+ cells exhibited cytoplasmic κ restriction. In addition to the clonal plasma cells, T-cells that co-expressed CD3 and CD4 were also found to be positive for the known T-cell antigens CD2 and CD5 but negative for CD7. Consistent with the immunohistological findings from primary lymph node, T-cells were found to be negative for CD10. T-cells with immunophenotypic variation were considered AITL cells and comprised up to 0.25% of the total gated events. Representative scatter plots of the immunophenotypic findings obtained from the flow cytometry analysis are shown in Fig. 2.
PCR analysis of the T-cell receptor gamma chain gene (TCRγ) demonstrated a clonal rearrangement that confirms T-cell clonality. Molecular analyses for the TCRγ rearrangement was performed through PCR by using a mixture of consensus V and J region primers. The PCR products were analyzed by nonradioactive single-strand confirmation polymorphism. Karyotypic analysis of the BM cell culture using conventional G-banding showed 46,XY in all metaphase cells analyzed (n=20).
Consequently, the patient was diagnosed as having BM involvement of AITL and plasma cell leukemia (IgMκ) simultaneously. Following initial diagnosis, the patient received the first cycle of chemotherapy consisting of vincristine, doxorubicin, cyclophosphamide, and prednisolone. The patient also suffered from respiratory failure combined with septic shock and was managed at the intensive care unit. Following discharge, the patient was followed up to monitor the disease in an outpatient clinic, without further chemotherapy.
AITL, one subtype of peripheral T-cell lymphoma, is frequently associated with polyclonal B-cell or plasma cell proliferation [1]. The B-cell expansion observed in AITL has been proposed to be related to the functions of neoplastic follicular helper T-cells. However, proliferation of clonal B-cells or plasma cells has rarely been reported [2, 3, 4]. We hypothesized that the clonal plasma cell population is unrelated to the patient's AITL status. In over 20% of clonal plasma cells in PB and BM, the presence of M-protein in serum and absence of EBV could support this possibility. Factors contradicting plasma cell neoplasm include the followings: negative organ impairment including hypercalcemia; renal insufficiency; anemia; bone lesions; and IgM paraprotein (non-IgG or IgA) that could be associated with a clone of lymphoplasmacytic cells [5]. However, there was no evidence of CD20+ neoplastic B-cells in BM.
In conclusion, the current case emphasizes that AITL could present with marked proliferation of clonal plasma cells, thereby potentially masking underlying T-cell lymphoma. Being aware of this possible effect is clinically important when determining treatment strategies and prognostic stratification, especially in the patients with a clonal B-cell population.
References
1. Grogg KL, Morice WG, Macon WR. Spectrum of bone marrow findings in patients with angioimmunoblastic T-cell lymphoma. Br J Haematol. 2007; 137:416–422. PMID: 17488486.

2. Huppmann AR, Roullet MR, Raffeld M, Jaffe ES. Angioimmunoblastic T-cell lymphoma partially obscured by an Epstein-Barr virus-negative clonal plasma cell proliferation. J ClinOncol. 2013; 31:e28–e30.

3. Balagué O, Martínez A, Colomo L, Roselló E, Garcia A, Martónez-Bernal M, et al. Epstein-Barr virus negative clonal plasma cell proliferations and lymphomas in peripheral T-cell lymphomas: a phenomenon with distinctive clinicopathologic features. Am J Surg Pathol. 2007; 31:1310–1322. PMID: 17721185.
4. Willenbrock K, Bräuninger A, Hansmann ML. Frequent occurrence of B-cell lymphomas in angioimmunoblastic T-cell lymphoma and proliferation of Epstein-Barr virus-infected cells in early cases. Br J Haematol. 2007; 138:733–739. PMID: 17672882.

5. Lin P, Hao S, Handy BC, Bueso-Ramos CE, Medeiros LJ. Lymphoid neoplasms associated with IgM paraprotein: a study of 382 patients. Am J Clin Pathol. 2005; 123:200–205. PMID: 15842043.
Fig. 1
Histologic findings of the bone marrow biopsy. (A) Bone marrow biopsy showed nodular lymphocytic infiltrates with malignant cells (Hematoxylin and Eosin, ×400). (B) The malignant cells were positive for CD3 (×400). (C, D) Plasma cells expressed κ light chain restriction. Immunohistochemical staining with anti-κ (C) and anti-λ (D) (×400).
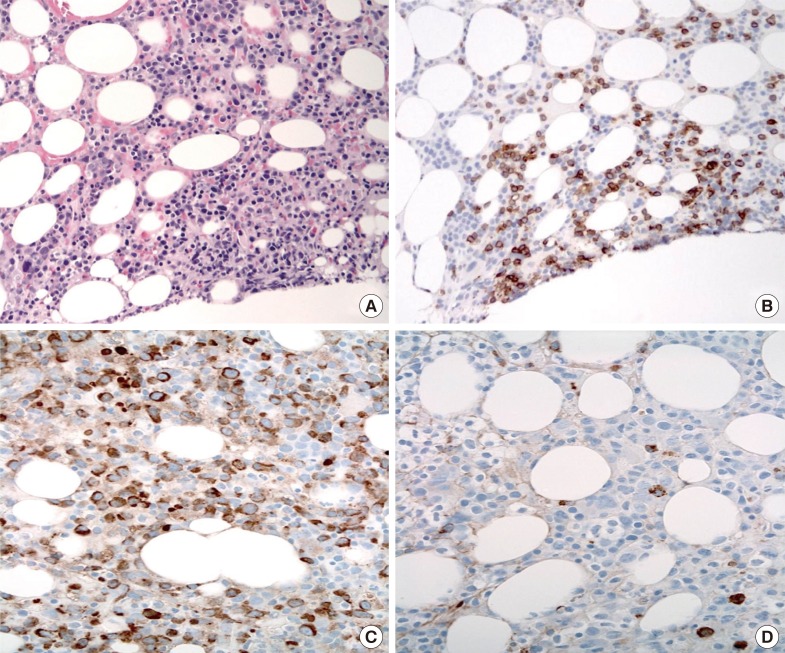
Fig. 2
Flow cytometry analysis of circulating lymphocytes. (A) and (B) CD45 and side scatter (SSC) gating: T-cells were positive for CD3, and B-cells were positive for CD19. (C) and (D) The CD19+ B-cells expressed high density of CD38 and cytoplasmic monoclonal immunoglobulin light chain κ proteins suggestive of plasma cells. (E) and (F) CD3+ T-cells were negative for CD7 and positive for CD4 suggesting the presence of malignant T-cells with immunophenotypic variation.
Abbreviations: SSC, side scatter; cKAPPA, cytoplasmic immunoglobulin κ; cLAMBDA, cytoplasmic immunoglobulin λ.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download