1. Blake KD, Davenport SL, Hall BD, Hefner MA, Pagon RA, Williams MS, et al. CHARGE association: an update and review for the primary pediatrician. Clin Pediatr (Phila). 1998; 37:159–173. PMID:
9545604.

2. Verloes A. Updated diagnostic criteria for CHARGE syndrome: a proposal. Am J Med Genet A. 2005; 133A:306–308. PMID:
15666308.

3. Woodage T, Basrai MA, Baxevanis AD, Hieter P, Collins FS. Characterization of the CHD family of proteins. Proc Natl Acad Sci USA. 1997; 94:11472–11477. PMID:
9326634.

4. Aramaki M, Udaka T, Kosaki R, Makita Y, Okamoto N, Yoshihashi H, et al. Phenotypic spectrum of CHARGE syndrome with CHD7 mutations. J Pediatr. 2006; 148:410–414. PMID:
16615981.

5. Zentner GE, Layman WS, Martin DM, Scacheri PC. Molecular and phenotypic aspects of CHD7 mutation in CHARGE syndrome. Am J Med Genet A. 2010; 152A:674–686. PMID:
20186815.
6. Jongmans MC, Admiraal RJ, van der Donk KP, Vissers LE, Baas AF, Kapusta L, et al. CHARGE syndrome: the phenotypic spectrum of mutations in the CHD7 gene. J Med Genet. 2006; 43:306–314. PMID:
16155193.

7. Sanlaville D, Verloes A. CHARGE syndrome: an update. Eur J Hum Genet. 2007; 15:389–399. PMID:
17299439.

8. Janssen N, Bergman JE, Swertz MA, Tranebjaerg L, Lodahl M, Schoots J, et al. Mutation update on the CHD7 gene involved in CHARGE syndrome. Hum Mutat. 2012; 33:1149–1160. PMID:
22461308.

9. Lee YW, Kim SC, Shin YL, Kim JW, Hong HS, Lee YK, et al. Clinical and genetic analysis of the CHD7 gene in Korean patients with CHARGE syndrome. Clin Genet. 2009; 75:290–293. PMID:
19159393.
10. Song MH, Cho HJ, Lee HK, Kwon TJ, Lee WS, Oh S, et al. CHD7 mutational analysis and clinical considerations for auditory rehabilitation in deaf patients with CHARGE syndrome. PLoS One. 2011; 6:e24511. PMID:
21931733.

11. Cho HJ, Song MH, Choi SY, Kim J, Lee J, Kim UK, et al. Genetic analysis of the CHD7 gene in Korean patients with CHARGE syndrome. Gene. 2013; 517:164–168. PMID:
23333604.

12. Kim Y, Lee HS, Yu JS, Ahn K, Ki CS, Kim J. Identification of a novel mutation in the CHD7 gene in a patient with CHARGE syndrome. Korean J Pediatr. 2014; 57:46–49. PMID:
24578717.
13. Hastings PJ, Lupski JR, Rosenberg SM, Ira G. Mechanisms of change in gene copy number. Nat Rev Genet. 2009; 10:551–564. PMID:
19597530.

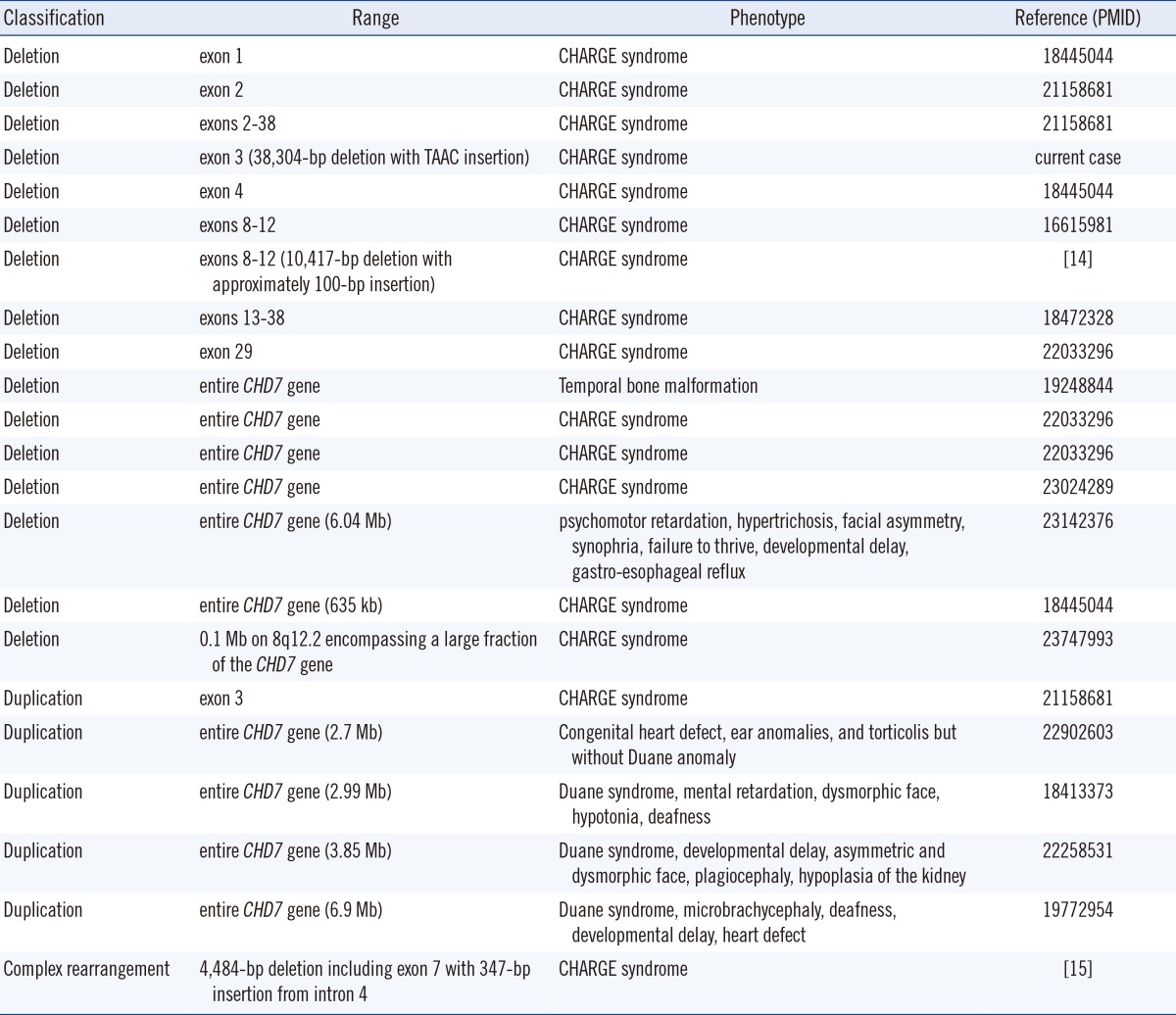
14. Udaka T, Okamoto N, Aramaki M, Torii C, Kosaki R, Hosokai N, et al. An Alu retrotransposition-mediated deletion of CHD7 in a patient with CHARGE syndrome. Am J Med Genet A. 2007; 143A:721–726. PMID:
17334995.
15. Vatta M, Niu Z, Lupski JR, Putnam P, Spoonamore KG, Fang P, et al. Evidence for replicative mechanism in a CHD7 rearrangement in a patient with CHARGE syndrome. Am J Med Genet A. 2013; 161A:3182–3186. PMID:
23956205.

16. Guirouilh-Barbat J, Huck S, Bertrand P, Pirzio L, Desmaze C, Sabatier L, et al. Impact of the KU80 pathway on NHEJ-induced genome rearrangements in mammalian cells. Mol Cell. 2004; 14:611–623. PMID:
15175156.

17. Roth DB, Porter TN, Wilson JH. Mechanisms of nonhomologous recombination in mammalian cells. Mol Cell Biol. 1985; 5:2599–2607. PMID:
3016509.

18. Roth DB, Proctor GN, Stewart LK, Wilson JH. Oligonucleotide capture during end joining in mammalian cells. Nucleic Acids Res. 1991; 19:7201–7205. PMID:
1662811.

19. Little KC, Chartrand P. Genomic DNA is captured and amplified during double-strand break (DSB) repair in human cells. Oncogene. 2004; 23:4166–4172. PMID:
15048077.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download