Abstract
Background
Major burn injuries induce inflammatory responses and changes in the levels of various cytokines. This study was conducted to assess early changes in the serum levels of inflammatory cytokines after burn injury, identify cytokines associated with mortality, and characterize correlations among cytokines.
Methods
Blood samples of 67 burn patients were collected on days 1 and 3 after burn injury, and the concentrations of 27 cytokines were measured using the Bio-Plex Suspension Array System (Bio-Rad Laboratories, USA). Blood samples of 25 healthy subjects were used as controls. We analyzed statistical differences in the concentrations of each cytokine between the control and patient groups, between day 1 and day 3, and between survival and nonsurvival groups. Correlations among 27 cytokines were analyzed.
Results
Median concentrations of granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), interleukin 1 receptor antagonist (IL-1RA), interleukin 6 (IL-6), interleukin 8 (IL-8), interleukin 10 (IL-10), interleukin 15 (IL-15), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1β (MIP-1β), and vascular endothelial growth factor (VEGF) were significantly higher in burn patients than in controls. IL-1RA, IL-6, and MCP-1 levels were significantly higher in the nonsurvival group than in the survival group on day 1 after burn injury. Correlation analysis of 27 cytokines showed different relationships with one another. Stronger correlations among interferon γ (IFN-γ), IL-2, IL-4, IL-7, IL-12p70, and IL-17 were found.
Major burn injury induces an inflammatory response that is characterized by the activation of inflammatory pathways and the release of various cytokines [1]. Inflammation is controlled by the balance between pro- and anti-inflammatory mediators in a complex cytokine network [2]. In this network, one cytokine can influence multiple cell types (pleiotropy), and multiple cytokines can have similar biological effects on the same cell type (redundancy) [3]. Hence, the action of one cytokine can be compensated by another cytokine. The uncontrolled release of pro- and anti-inflammatory cytokines promotes immunological dysfunction and significant systemic inflammation, which result in tissue damage, multiple organ failure, or death in burn patients [1].
Although there have been several studies on various cytokines in burn patients [1, 4, 5, 6, 7, 8, 9, 10, 11], the pathogenic and prognostic role of each cytokine has not been fully elucidated. The purpose of the present study was to assess early changes in the serum levels of inflammatory cytokines after burn injury, identify cytokines associated with mortality, and characterize correlations among various cytokines.
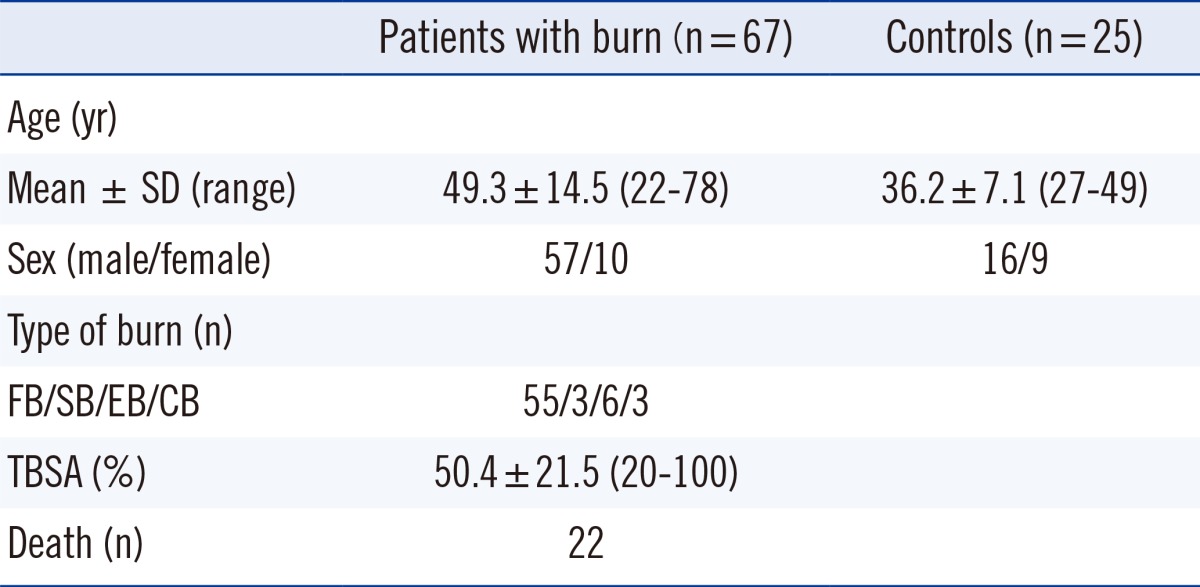
Sixty-seven burn patients and 25 healthy subjects participated in this prospective study from May 2011 to June 2012. Patients that were included in the study met the following criteria: individuals aged over 18 yr having arrived at a participating hospital within 24 hr after the injury with the total burn surface area (TBSA) greater than 20%. The patients' clinical data, such as those pertaining to age, sex, type of burn, TBSA, and hospital course were collected. The patients had a mean age of 49.3 yr (range, 22-78 yr) and 57 patients (85.1% of 67 patients) were men (Table 1). Flame burn was the major type of burn (flame burn/scalding burn/electrical burn/chemical burn=59/3/6/3). TBSA ranged from 20% to 100% (mean±SD: 50.4%±21.5%), and 22 of 67 patients (32.8%) died. We classified the patients as survivors or non-survivors according to their mortality at least 3 months later after burn injury. Serum samples from 25 healthy adults (age: 27-49 yr) were collected as the control. The study protocols were approved by the Institutional Review Board of the Hangang Sacred Heart Hospital (IRB no. 2010-104), and written informed consent was obtained from each patient or a qualified family member.
Blood samples were collected from the patients on arrival (day 1) and on day 3 after the burn injury, and blood samples from 25 healthy non-burn subjects were used as controls. Blood was collected in a SST tube (Becton Dickinson, Franklin Lakes, NJ, USA) and was processed within 30 min of collection. The tubes were centrifuged at 2,265 g for 10 min, after which the serum was stored in aliquots at -70℃ until analysis. Concentrations of granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), interleukin 1 receptor antagonist (IL-1RA), interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 6 (IL-6), interleukin 7 (IL-7), interleukin 8 (IL-8), interleukin 9 (IL-9), interleukin 10 (IL-10), interleukin-12 (IL-12) p70, interleukin 13 (IL-13), interleukin 15 (IL-15), interleukin 17 (IL-17), IP-10, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), macrophage inflammatory protein 1β (MIP-1β), platelet-derived growth factor (PDGF)-BB, regulated on activation, normal T cell expressed and secreted (RANTES), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (FGF), and eotaxin were measured using the Bio-Plex Pro human cytokine 27-plex assay (Bio-Rad Laboratories, Hercules, CA, USA) with the Bio-Plex Suspension Array System (Bio-Rad Laboratories), according to the manufacturer's instructions. In brief, serum samples were thawed, centrifuged at 2,265 g for 5 min, and then incubated with microbeads labeled with antibodies specific to the above-mentioned cytokines for 30 min. After the wash step, the beads were incubated with the detection antibody cocktail, with each antibody specific to a single cytokine. After another wash step, the beads were incubated with streptavidin-phycoerythrin for 10 min and washed again, and the concentration of each cytokine was then determined by using the Bio-Plex Suspension Array Reader.
The data were calculated as mean±SD or median (range). Data missing because of a patient's death were excluded from the calculations of mean±SD and median (range). Statistical differences in the concentrations of each cytokine between the control and the patient group, between day 1 and day 3, and between the survival and nonsurvival groups were analyzed by using the Mann-Whitney test. Spearman correlation coefficients (r) were calculated to evaluate the relationship among cytokine concentrations. We used the arbitrary criteria of the value of correlation coefficients (r) to show the level of correlation (r>0.8; 0.6<r≤0.8; 0.4<r≤0.6; r≤0.4). The differences were considered statistically significant when P<0.05. The MedCalc version 12.7.4.0 (MedCalc Software, Ostend, Belgium) was used for statistical analysis.
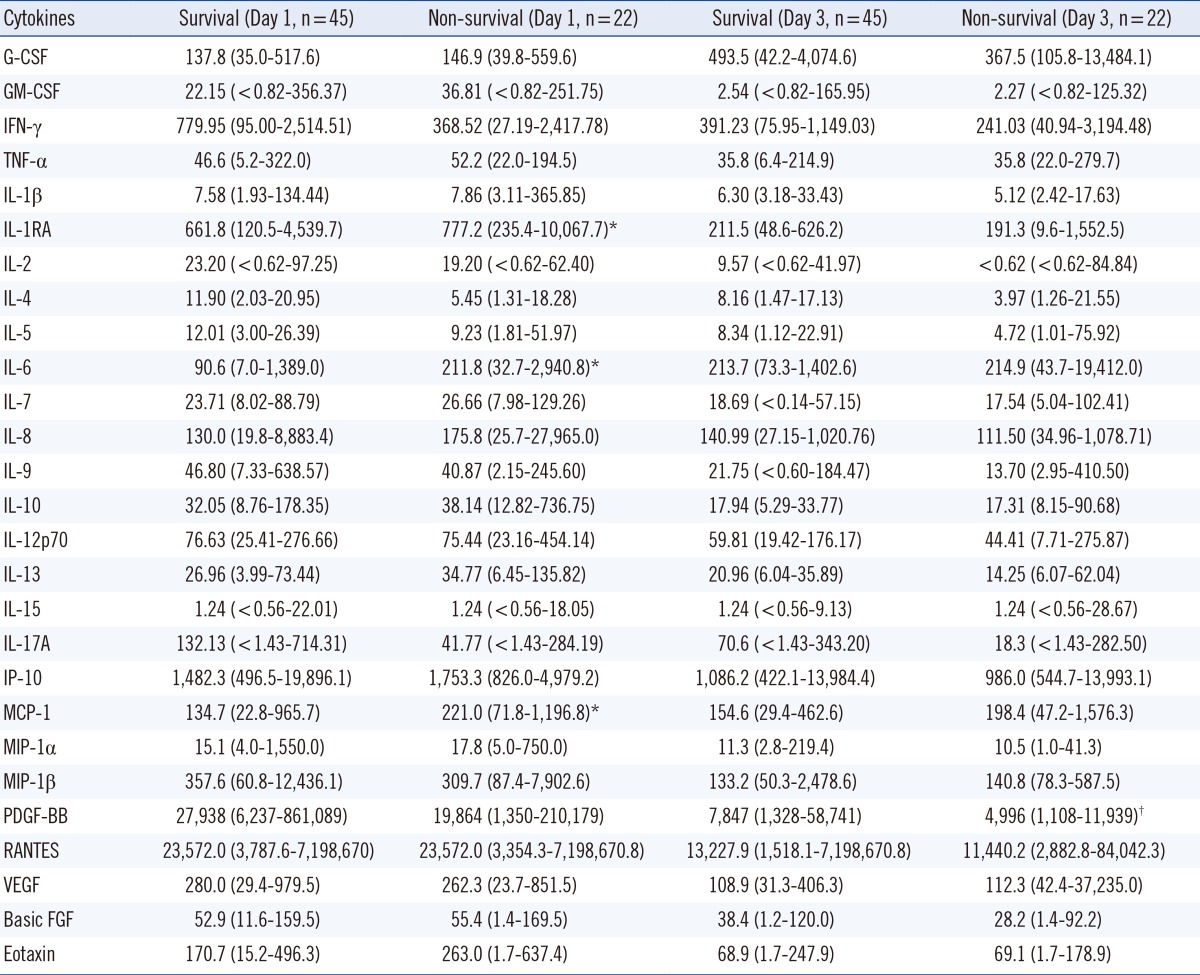
Compared to the serum concentrations for the control subjects, serum concentrations of G-CSF, GM-CSF, IL-1RA, IL-6, IL-8, IL-10, IL-15, MCP-1, MIP-1β, and VEGF were significantly higher in burn patients (P<0.05) (Table 2). The median concentrations of GM-CSF, IFN-γ, TNF-α, IL-1RA, IL-2, IL-4, IL-5, IL-7, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-17A, IP-10, MIP-1α, MIP-1β, PDGF-BB, RANTES, VEGF, basic FGF, and eotaxin were significantly higher on day 1 than on day 3, whereas the opposite was true for G-CSF and IL-6 (P<0.05). Serum levels of IL-1RA, IL-6, and MCP-1 were significantly different between the nonsurvival and the survival groups on day 1 after burn injury (P<0.05), whereas for most cytokines, the concentrations were not different between the two groups, either on day 1 or on day 3 after burn injury (Table 3).
Correlation coefficients (r) between cytokine levels in burn patients are shown in Fig. 1, and only significant coefficients (P<0.05, Spearman correlation test) are shown. Correlation analysis of 27 cytokines revealed different levels of correlation with one another. Stronger correlations among IFN-γ, IL-2, IL-4, IL-7, IL-12p70, and IL-17 cytokines were observed.
In this study, the serum concentrations of G-CSF, GM-CSF, IL-1RA, IL-6, IL-8, IL-10, IL-15, MCP-1, MIP-1β, and VEGF were significantly higher in burn patients than in healthy subjects. Several studies have been published on the changes in cytokine levels in burn patients. The common finding in these previous studies and in our study is that serum levels of IL-6, IL-8, MCP-1, and G-CSF are significantly increased in burn patients [1, 4, 5, 6]. However, the concentrations of GM-CSF, IFN-γ, TNF-α, IL-1β, IL-1RA, IL-2, IL-4, IL-5, IL-7, IL-10, IL-12, IL-13, IL-15, IL-17, MIP-1β, and VEGF were found to be increased in some studies but not in others [1, 4, 5, 6]. This discrepancy could be explained as follows: cytokine concentrations fluctuate in seriously ill patients and are influenced by multiple factors, such as age, burn size, time after burn injury, comorbidities or complications, and the white blood cell count. The upregulated cytokines could be mediators induced directly by the burn, secondary mediators, or merely the markers of systemic inflammation or other concomitant problems. It is currently difficult to distinguish among these possibilities. Of the upregulated cytokines, IL-6 and IL-8 are well-known proinflammatory cytokines, whereas IL-10 and IL-1RA are anti-inflammatory (immunomodulatory) cytokines. These data suggest that both proinflammatory and anti-inflammatory activities are involved in early burn injury. Our finding that not all proinflammatory cytokines are upregulated in burn patients suggests that some kinds of a system of checks and balances in the cytokine network is maintained in these patients.
In the correlation analysis among the cytokines, we could identify that the levels of many cytokines had a different relationship with one another and stronger correlations were observed among IFN-γ, IL-2, IL-4, IL-7, IL-12p70, and IL-17 (r>0.8, P<0.0001) (Fig. 1). Although the exact mechanism of those relationships is currently unknown, IFN-γ, IL-2, IL-4, IL-7, IL-12p70, and IL-17 are often secreted by T helper cells. It is likely that after a burn injury, the increase in cytokine concentrations in the blood is induced by interactions within a complex network of cytokine-related pathways rather than by a single factor.
Most cytokines in this study were upregulated more on day 1 than on day 3 after the burn injury, except for G-CSF and IL-6. Previous studies showed varying results for the change in cytokine levels, depending on the postburn time [1, 5, 8, 9, 10, 11]; the time to peak concentration of each cytokine varies among individuals and among studies. These divergent results may be explained as follows: gene expression of cytokines is influenced by multiple factors, including age, burn size, comorbidities or complications, and the white blood cell count. The different behavior of cytokines is suggestive of the distinct and different roles of each cytokine in modulating immune (including inflammatory) responses after severe burns.
Regarding mortality, there were significantly different levels of IL-1RA, IL-6, and MCP-1 between the nonsurvival and the survival groups on day 1 after burn injury. Our results are in line with a recent study [6], where IL-1RA was most predictive of mortality, and IL-6, IL-8, MCP-1, IL-15, and eotaxin were also related to mortality. In other studies, IL-6 and IL-10 were related to mortality [7, 8]. These results suggest that some cytokines may be used as a prognostic marker in burn injury.
In summary, the serum levels of G-CSF, GM-CSF, IL-1β, IL-1RA, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-13, MCP-1, MIP-1α, MIP-1β, and VEGF increase after burn injury, and most cytokines, except for G-CSF and IL-6, are upregulated more on day 1 than on day 3. The concentrations of IL-1RA, IL-6, and MCP-1 were higher in the nonsurvival group than in the survival group on day 1 after burn injury. Strong correlations among IFN-γ, IL-2, IL-4, IL-7, IL-12p70, and IL-17 cytokines were found; these data suggest that IL-1RA, IL-6, and MCP-1 may serve as a prognostic indicator in patients with burn injury, and the increase in cytokine concentrations is induced by interactions within a complex network of cytokine-related pathways rather than by a single factor.
Acknowledgments
This study was supported by a grant from Hallym University Medical Center Research Fund (01-2010-11). We really appreciate Hae Won Kwon for excellent technical support and data collection.
References
1. Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DM, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006; 26:13–19. PMID: 16783192.

2. Elias JA, Freundlich B, Kern JA, Rosenbloom J. Cytokine networks in the regulation of inflammation and fibrosis in the lung. Chest. 1990; 97:1439–1445. PMID: 2112081.

3. Ozaki K. Cytokine and cytokine receptor pleiotropy and redundancy. J Biol Chem. 2002; 277:29355–29358. PMID: 12072446.

4. Mikhal'chik EV, Piterskaya JA, Budkevich LY, Pen'kov LY, Facchiano A, De Luca C, et al. Comparative study of cytokine content in the plasma and wound exudate from children with severe burns. Bull Exp Biol Med. 2009; 148:771–775. PMID: 20396789.
5. Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, et al. Long-term persistence of the pathophysiologic response to severe burn injury. PLoS ONE. 2011; 6:e21245. PMID: 21789167.
6. Davis CS, Janus SE, Mosier MJ, Carter SR, Gibbs JT, Ramirez L, et al. Inhalation injury severity and systemic immune perturbations in burned adults. Ann Surg. 2013; 257:1137–1146. PMID: 23160150.

7. Gauglitz GG, Finnerty CC, Herndon DN, Mlcak RP, Jeschke MG. Are serum cytokines early predictors for the outcome of burn patients with inhalation injuries who do not survive? Crit Care. 2008; 12:R81. PMID: 18564432.

8. Csontos C, Foldi V, Pálenkas L, Bogar L, Röth E, Weber G, et al. Time course of pro- and anti-inflammatory cytokine levels in patients with burns-prognostic value of interleukin-10. Burns. 2010; 36:483–494. PMID: 20045261.

9. Kim HS, Kim JH, Yim H, Kim D. Changes in the levels of interleukins 6, 8, and 10, tumor necrosis factor alpha, and granulocyte-colony stimulating factor in Korean burn patients: relation to burn size and postburntime. Ann Lab Med. 2012; 32:339–344. PMID: 22950069.
10. Finnerty CC, Jeschke MG, Herndon DN, Gamelli R, Gibran N, Klein M, et al. Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med. 2008; 14:553–560. PMID: 18548133.

11. Lantos J, Földi V, Roth E, Wéber G, Bogár L, Csontos C. Burn trauma induces early HMGB1 release in patients: its correlation with cytokines. Shock. 2010; 33:562–567. PMID: 19997053.
Fig. 1
Correlations among 27 cytokines in the serum samples of burn patients (n=134). Correlation coefficients (r) corresponding to significant correlations (P<0.05) are presented. Upper line number in each box indicates correlation coefficient (r) and lower line number indicates P value. Red color indicates the strongest correlation (r>0.8) and green color (0.6<r≤0.8) and yellow color (0.4<r≤0.6) indicates stronger correlation.
Abbreviation: NS, not-significant.
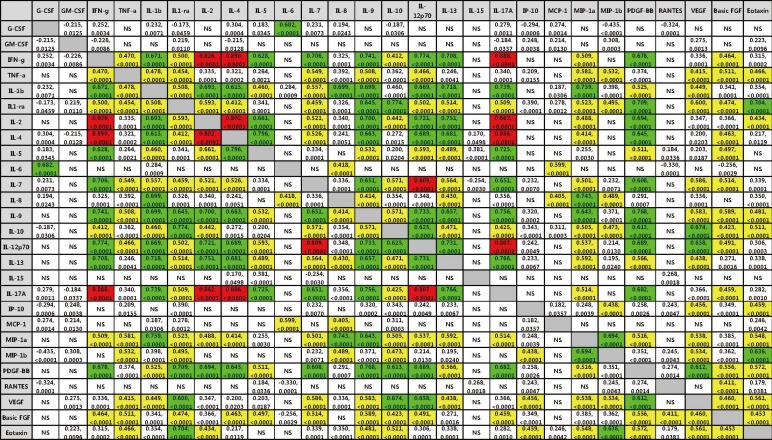
Table 2
Serum concentrations of various cytokines in controls and burn patients on days 1 and 3 after burn injury
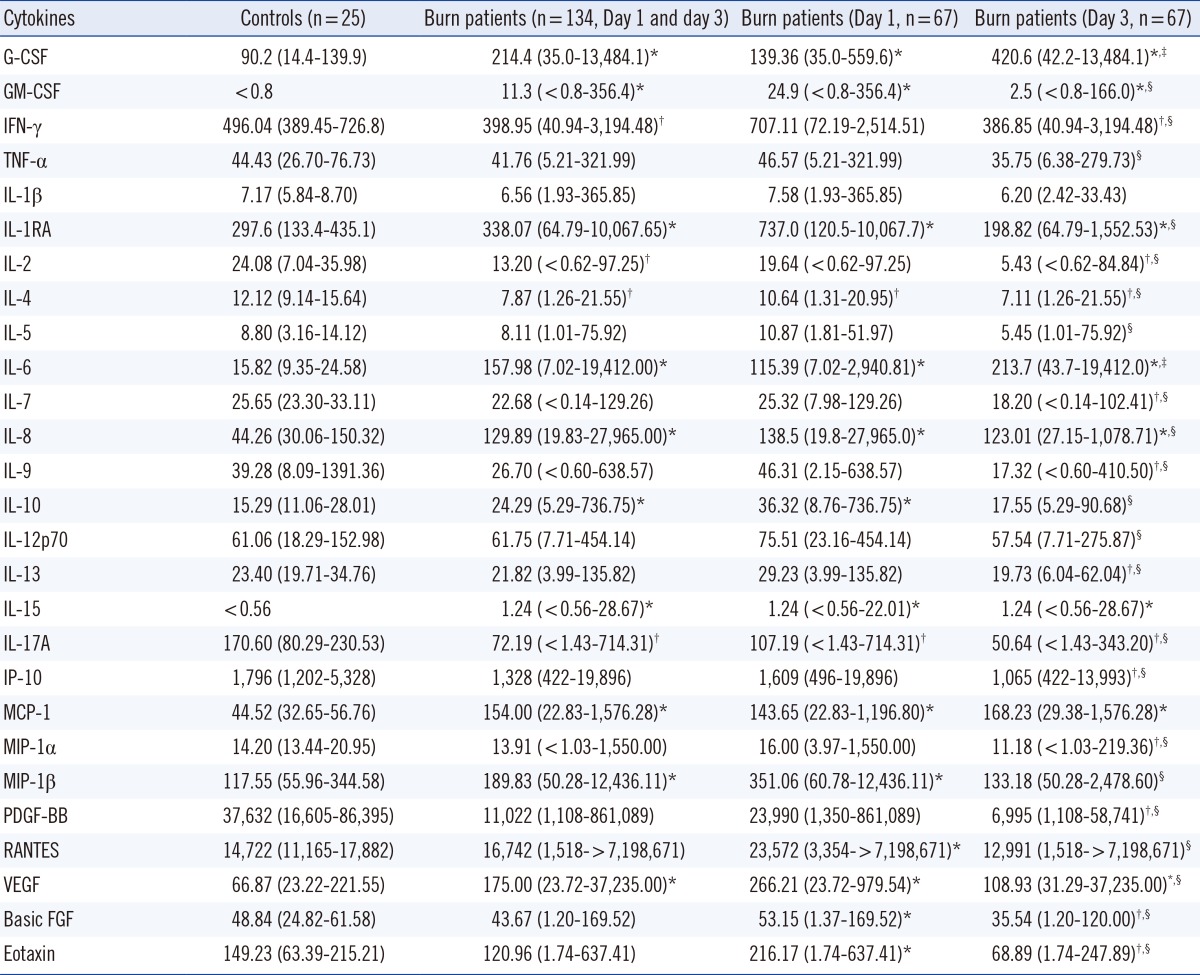
Data were expressed as the median (range) in pg/mL.
*Significant increase in burn patients compared to controls (P<0.05); †Significant decrease in burn patients compared to controls (P<0.05); ‡Significant increase on Day 3 compared to Day 1 (P<0.05); §Significant decrease on Day 3 compared to Day 1 (P<0.05).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download