Abstract
Lung disease caused by nontuberculous mycobacteria (NTM) represents an increasing proportion of all mycobacterial diseases. We investigated recent occurrences of NTM and evaluated the clinical significance of NTM isolates from 752 respiratory specimens collected from patients at National Health Insurance Service Ilsan Hospital between January 2007 and May 2011. Specimens were incubated on solid and liquid media (BACTEC MGIT 960, BD, USA) for 6-8 weeks, and PCR and reverse blot hybridization were performed (REBA Myco-ID, Molecules & Diagnostics, Korea). Clinical features of the patients were reviewed through medical records. The most frequently isolated organism was Mycobacterium avium (46.7%), followed by M. intracellulare (14.8%), M. fortuitum (7.2%), and M. abscessus (6.6%). The most common mycobacteria among definitive cases of NTM lung disease were M. avium (42/351, 12.0%), M. intracellulare (19/111, 17.1%), M. abscessus (11/50, 22.0%), M. massiliense (4/13, 30.8%), and M. fortuitum (4/54, 7.4%). Clinically significant cases of NTM lung disease increased from 4 patients in 2007 to 32 in 2011. The mean patient age was 64 yr (range: 35-88 yr), and 58 (64%) patients were women. Patients suffered from cough, productive sputum, and hemoptysis. In summary, the most common mycobacteria causing NTM lung disease were M. avium and M. intracellulare; however, cases of M. massiliense and M. abscessus infection are on the rise in Korea.
The prevalence of nontuberculous mycobacterium (NTM) infection and NTM lung disease have increased worldwide since the 1950s [1, 2]. In Korea, a region with an intermediate tuberculosis burden, the incidence of NTM lung disease was 1.82 per 100,000 patients (inpatients and outpatients) in 2006, and increased to 4.38 per 100,000 in 2010 [3]. In contrast, in the U.S, the prevalence rose from 1.4 to 6.6 per 100,000 between 2004 and 2006, and was higher in women than in men [4]. NTM causes opportunistic infection, and pulmonary disease is its most common localized clinical manifestation. Other manifestations include disseminated disease and diseases of the lymphatic system, skin, soft tissue, and bone [5, 6]. The symptoms of NTM pulmonary disease are variable and nonspecific [6]; however, nearly all patients have chronic cough and other symptoms, such as sputum production, hemoptysis, dyspnea, fatigue, malaise, fever, chest pain, and weight loss [5]. Radiographic features of NTM lung disease are primarily fibrocavitary, characterized by nodules and bronchiectasis. However, these are not sufficient to exclude a diagnosis of tuberculosis [7, 8]. As such, it is difficult to diagnose pulmonary disease arising from NTM, especially in countries with high tuberculosis prevalence rates. Koh et al. [9] reported that about one-quarter of the patients examined between 2002 and 2003, from whose respiratory specimens NTM was isolated, had clinically significant NTM lung infections. The aims of the present study were to investigate the recent incidences and clinical significance of NTM isolates collected from patients of a hospital in Korea over a 4-yr period.
All NTM isolates were recovered from respiratory specimens such as sputum, bronchial washing or lavage fluid, and lung tissue of patients undergoing treatment at the National Health Insurance Service Ilsan Hospital between January 2007 and May 2011. Clinical features of patients from whom NTM isolates were taken more than twice within a 6-month period, and more than once by bronchial washing or lavage and lung biopsy, were tracked through electronic medical records. The protocol was reviewed and approved by the Institutional Review Board of National Health Insurance Service, Ilsan Hospital.
All respiratory specimens were decontaminated with an N-acetyl-L-cysteine-2% sodium hydroxide solution, inoculated on solid Ogawa media and/or liquid media (BACTEC MGIT 960, BD, Sparks, MD, USA) and incubated for 6-8 weeks. Ogawa media only was used until September 2010; thereafter, both types of media were used. Ziehl-Neelsen staining for acid-fast bacilli (AFB) was performed on samples grown on both types of media. For NTM species identification, PCR and reverse blot hybridization were performed using material obtained from colonies or from liquid media (REBA Myco-ID, Molecules & Diagnostics, Wonju, Korea) according to manufacturer's instructions. For further identification, PCR and sequencing of the rpoB gene were carried out at a commercial laboratory (Seoul Clinical Laboratories, Seoul, Korea).
The American Thoracic Society (ATS) issued a revised set of diagnostic criteria for NTM lung disease in 2007, summarized as follows: clinical criteria are 1) pulmonary symptoms, nodular or cavitary opacities on chest radiographs, or a high-resolution computed tomography scan that shows multifocal bronchiectasis with multiple small nodules; and 2) appropriate exclusion of other diagnoses. Microbiological criteria are 1) positive culture results from at least 2 separate expectorated sputum samples; 2) positive culture results from at least one bronchial wash or lavage; and 3) transbronchial or other lung biopsies with mycobacterial histopathological features and a positive culture for NTM, and one or more sputum or bronchial washings that are culture positive for NTM. The ATS guidelines also advise that 1) expert consultation should be obtained when rare NTM are encountered, which may result from environmental contamination, 2) patients who are suspected to have NTM lung disease but who do not meet the diagnostic criteria should be followed until a diagnosis is established or excluded, and 3) a positive diagnosis of NTM lung disease does not necessitate therapy, which is a decision based on the potential risks and benefits of therapy for individual patients [5].
A total of 752 respiratory specimens (697 sputum, 53 bronchial washing or lavage, and 2 lung tissue) were collected from patients between January 2007 and May 2011. Clinically significant cases of NTM lung disease increased from 4 patients in 2007 to 32 patients in 2011. The most frequently isolated organism was M. avium (46.7%), followed by M. intracellulare (14.8%), M. fortuitum (7.2%), and M. abscessus (6.6%) (Table 1). The choice of medium (solid or liquid) had no effect on the occurrence of a given NTM species. However, the frequency of NTM isolates increased after liquid medium was added to the protocol in 2010 (45 isolates/month) compared to 15 isolates/month when solid medium only was used from 2006 to 2010. The most frequently observed mycobacteria in definitive NTM lung disease were M. avium (n=42), and M. intracellulare (n=19), M. abscessus (n=11), M. massiliense (n=4), and M. fortuitum (n=4) were followed. Collectively, M. avium, M intracellulare, and M. abscessus were implicated in 80% (72/90) of the cases. Among patients from whom M. massiliense was isolated, 30.8% (4/13) showed definitive NTM lung infection, compared to 12.0% (11/50) of those infected with M. abscessus.
In 99 patients, NTM was detected twice or more within a span of 6 months. Approximately 66% (65/99) of patients from whom the same NTM species was isolated from sputum two or more times, and 46% (25/54) of those from whom NTM was isolated one or more times from bronchial wash or lavage, or lung biopsy in the same interval satisfied the 2007 diagnostic criteria for nontuberculous mycobacterial disease set forth by the ATS/Infectious Diseases Society of America (IDSA). The mean age of 90 patients with clinically significant NTM lung infections was 64 yr (range: 35-88 yr); 32 (35.6%) patients were men, and 58 (64.4%) were women. Forty nine (54.4%) patients complained of sputum production, 47 (52.2%) were listed as having chronic cough as one of their presenting symptoms, 29 (32.2%) exhibited hemoptysis at some point in their clinical course, although no massive hemoptysis was reported, 10 (11.1%) suffered from dyspnea, and 3 (3.3%) had chest pain. Seventeen (18.9%) patients had constitutional complaints, including weight loss (n=8; 8.9%), fever (n=6; 6.7%), fatigue (n=5; 5.6%), and malaise (n=2, 2.2%). Twenty patients (22.2%) had at least one positive AFB smear from their sputum or bronchial washing fluid.
In the United States and Japan, a M. avium complex (MAC) consisting of M. avium and M. intracellulare is the most common cause of NTM lung disease, followed by M. kansasii [1, 10, 11]. In England, M. kansasii is the most common pathogen associated with NTM lung disease [12]. The present study showed that MAC was the most common cause of disease in the Korean patients (61.5%), and that M. kansasii was responsible for only 2.9% of cases. While M. kansasii has been rarely observed in our studies [3, 9], another report suggests that cases involving M. kansasii have increased, especially in highly industrialized areas of Korea [13]. The rate of isolation of M. massiliense, previously grouped with M. abscessus, has been increasing in Japan, and patients with M. massiliense infections reportedly respond more favorably to antimicrobial therapy than those infected with M. abscessus [14]. In the present study, M. massiliense was detected in 1.7% of NTM isolates. It is noteworthy that 30.8% (4/13) of patients with definitive NTM lung infection tested positive for M. massiliense, whereas 12.0% (42/351) tested positive for M. avium. Therefore, it may be useful to exercise greater vigilance on cases of M. massiliense than on those of other NTM species. Although M. mucogenium is generally considered a contaminant when isolated from respiratory specimens, in about 4.0% of cases, the presence of M. mucogenium has been shown to be clinically significant [15]. M. mucogenium was found in 2.7% of NTM isolates, representing 5.0% of cases of NTM lung disease in this study. M. gordonae is frequently encountered in clinical laboratories but is almost always considered nonpathogenic [16]. In the present study, M. gordonae was found in 3.9% of NTM isolates, with 1 case being clinically significant.
In this study, 12.0% (90/752) of patients satisfied the 2007 ATS/IDSA diagnostic criteria for NTM disease; however, this is likely an under estimation due to the loss of patients during follow-up, or the presentation of only mild pulmonary symptoms such as cough and sputum production. Moreover, since the incidence of NTM isolates increased with the introduction of a liquid culture method during the survey period, the actual number of occurrences prior to this alteration was probably higher than what was detected.
In conclusion, the most common etiologies of NTM lung disease are infections by M. avium and M. intracellulare; however, the incidence of infections by M. massiliense and M. abscessus is rising in Korea.
Acknowledgements
This study was supported by a grant (no. 2012-31) from the National Health Service Ilsan Hospital Project.
References
1. O'Brien RJ, Geiter LJ, Snider DE Jr. The epidemiology of nontuberculous mycobacterial diseases in the United States. Results from a national survey. Am Rev Respir Dis. 1987; 135:1007–1014. PMID: 3579001.
2. Yates MD, Pozniak A, Uttley AH, Clarke R, Grange JM. Isolation of environmental mycobacteria from clinical specimens in south-east England: 19731993. Int J Tuberc Lung Dis. 1997; 1:75–80. PMID: 9441063.
3. Lee SK, Lee EJ, Kim SK, Chang J, Jeong SH, Kang YA. Changing epidemiology of nontuberculous mycobacterial lung disease in South Korea. Scand J Infect Dis. 2012; 44:733–738. PMID: 22720876.

4. Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010; 182:970–976. PMID: 20538958.

5. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007; 175:367–416. PMID: 17277290.

6. Saleeb P, Olivier KN. Pulmonary nontuberculous mycobacterial disease: new insights into risk factors for susceptibility, epidemiology, and approaches to management in immunocompetent and immunocom-promised patients. Curr Infect Dis Rep. 2010; 12:198–203. PMID: 21308530.

7. Moore EH. Atypical mycobacterial infection in the lung: CT appearance. Radiology. 1993; 187:777–782. PMID: 8497629.

8. Primack SL, Logan PM, Hartman TE, Lee KS, Müller NL. Pulmonary tuberculosis and Mycobacterium avium-intracellulare: a comparison of CT findings. Radiology. 1995; 194:413–417. PMID: 7824720.

9. Koh WJ, Kwon OJ, Jeon K, Kim TS, Lee KS, Park YK, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006; 129:341–348. PMID: 16478850.

10. Marras TK, Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med. 2002; 23:553–567. PMID: 12370992.
11. Sakatani M. The non-tuberculous mycobacteriosis. Kekkaku. 2005; 80:25–30. PMID: 15839060.
12. British Thoracic Society. Management of opportunist mycobacterial infections: Joint Tuberculosis Committee Guidelines 1999. Thorax. 2000; 55:210–218. PMID: 10679540.
13. Yim JJ, Park YK, Lew WJ, Bai GH, Han SK, Shim YS. Mycobacterium kansasii pulmonary diseases in Korea. J Korean Med Sci. 2005; 20:957–960. PMID: 16361804.
14. Harada T, Akiyama Y, Kurashima A, Nagai H, Tsuyuguchi K, Fujii T, et al. Clinical and microbiological differences between Mycobacterium abscessus and Mycobacterium massiliense lung diseases. J Clin Microbiol. 2012; 50:3556–3561. PMID: 22915613.
15. Wallace RJ Jr, Silcox VA, Tsukamura M, Brown BA, Kilburn JO, Butler WR. Clinical significance, biochemical features, and susceptibility patterns of sporadic isolates of the Mycobacterium chelonae-like organism. J Clin Microbiol. 1993; 31:3231–3239. PMID: 8308116.
16. Aguado JM, Gómez-Garcés JL, Manrique A, Soriano F. Pulmonary infection by Mycobacterium gordonae in an immunocompromised patient. Diagn Microbiol Infect Dis. 1987; 7:261–263. PMID: 3677577.
Table 1
Number of nontuberculous mycobacteria isolates recovered from patients at a hospital in Korea from January 2007 to May 2011
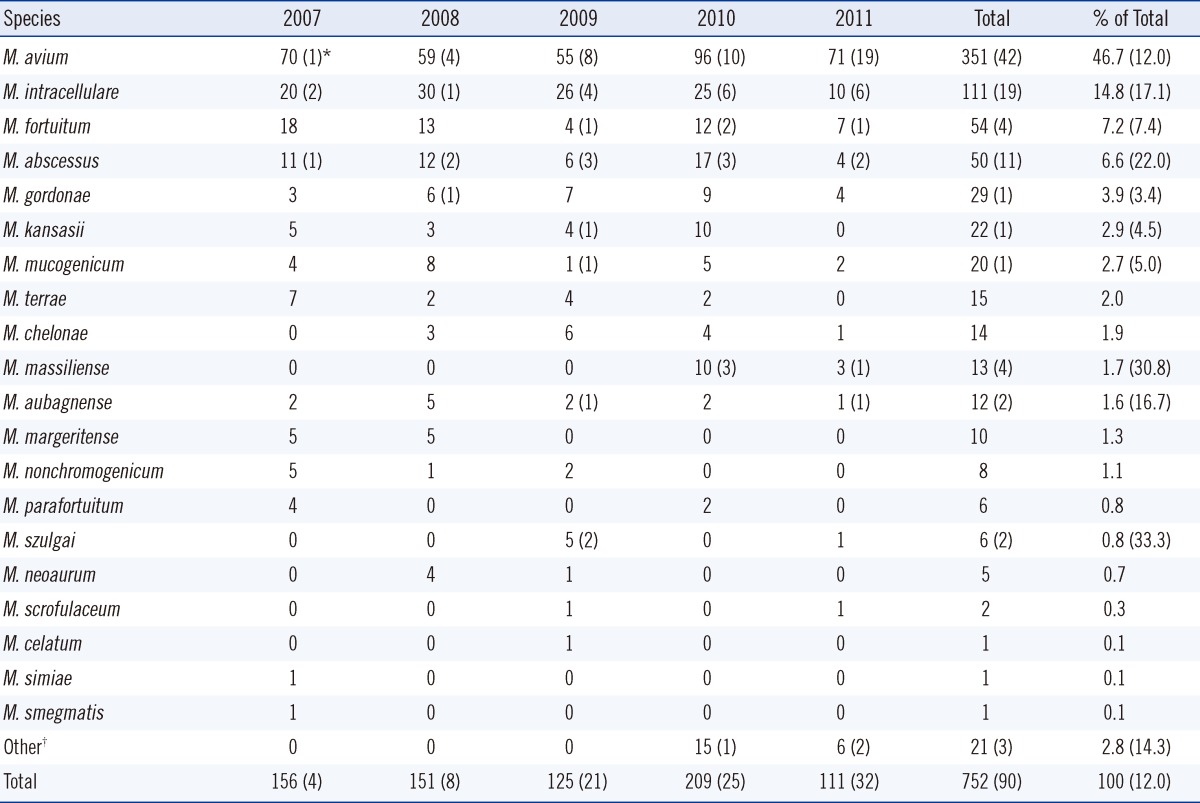
*Number in parentheses indicates the number of clinically significant cases; †M. avium/M. mucogenicum (n=5), M. avium/M. intracellulare (n=2), M. avium/M. abscessus (n=1), M. intracellulare/M. mucogenicum (n=3), M. terrae/M. nonchromogenicum (n=3), M. avium/M. fortuitum (n=2), M. gordonae/M. mucogenicum (n=1), M. gordonae/M. szulgai (n=1), M. intracellulare/M. scrofulaceu (n=1), M. kansasii/M. aubagnense (n=1), M. intracellulare/M. aubagnense (n=1).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download