Dear Editor
Plasmacytoma, a neoplastic proliferation of plasma cells, is a type of plasma cell dyscrasia that may manifest as multiple myeloma, primary amyloidosis, or monoclonal gammopathy of unknown significance. Extramedullary plasmacytoma rarely involves the mediastinum [1]. Simultaneous detection of mediastinal plasmacytoma and myelomatous pleural effusion accompanying myeloma is very rare. Furthermore, an amylase-producing multiple myeloma appears to be a fairly unusual phenomenon. This is a rare case of simultaneous extramedullary plasmacytoma and myelomatous pleural effusion with increased salivary-type amylase levels without evidence of pancreatic or salivary gland involvement. This is the first reported case of mediastinal plasmacytoma and myelomatous pleural effusion as an initial presentation accompanying a salivary-type amylase-producing multiple myeloma in Korea.
A 65-yr-old man was admitted with an anterior chest mass associated with dyspnea and nausea. Physical examination showed a 9×9 cm suprasternal mass with diffuse borders. Chest computed tomography (CT) showed an anterior mediastinal mass with anterior chest wall invasion and right pleural effusion and subsequent bilateral involvement. Provisional diagnosis of mediastinal lymphoproliferative disorder with malignant pleural effusion was made. The pleural effusion was tapped and found to be an exudate with an unusually high level of pleural fluid amylase, 5,809.7 IU/L, >4.4 times the serum value. Cytospin analysis of pleural effusion showed a proliferation of medium-sized oval cells with eccentric nuclei, abundant basophilic cytoplasm, and perinuclear halo, resembling plasma cells (Fig. 1).
The patient underwent an ultrasonography-guided biopsy of the anterior chest wall mass. Histologically, the tumor was characterized by a well-circumscribed proliferation of plasma cells that was positive for CD138 and lambda light chain on immunohistochemical stains (Fig. 1). The patient's mediastinal plasmacytoma was diagnosed at this time. There were no osteolytic regions on cranial, spinal, and pelvic radiographs. Serum protein electrophoresis and immunoelectrophoresis were unremarkable, but urine protein electrophoresis demonstrated a monoclonal peak in the beta immunoglobulin region, and urinary immunoelectrophoresis including IgD and IgE revealed an abnormal arc in the lambda light chain. Bone marrow aspiration and biopsy demonstrated hypercellular marrow with moderate neoplastic plasma cell proliferation (35%), consistent with plasma cell myeloma (Fig. 1). Immunohistochemical staining of the bone marrow specimen also showed neoplastic plasma cells positive for CD138, lambda light chain, and CD56. Chromosome analysis of the bone marrow specimen revealed 41,X,-Y,i(1)(q10),-4,-10,-16,-22[6]/42,idem,+add(16)(q24)[7]/46,XY[17]. Due to lack of bone marrow specimens, initial FISH profile for myeloma including IgH/CCND1 rearrangement, IgH/MAF rearrangement, IgH/FGFR3 rearrangement, 1q21 abnormalities, TP53 loss, and 13q14 loss was not performed. Karyotypic results showing hypodiploidy and 1q abnormalities could be suggestive of poor prognosis of tumor progression and advanced disease status.
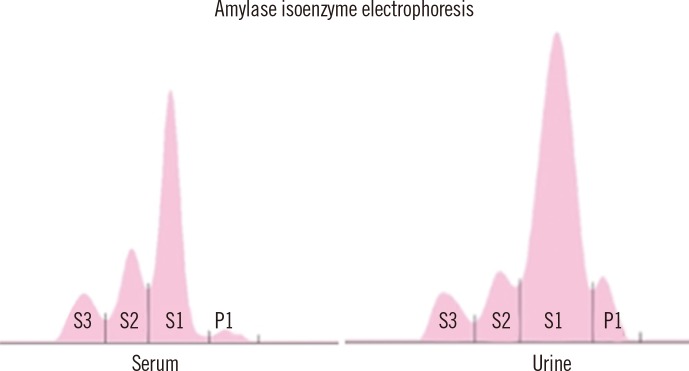
To study the increased amylase level in the pleural fluid, serum and urine amylase levels were measured. Amylase levels were up to 1,312.1 IU/L (reference range, 36-128 IU/L) in serum and 7,507.5 IU/L (reference range, 0-1,500 IU/L) in urine. Abdominal ultrasonography and CT revealed no significant pancreatic abnormality. The serum lipase level was within reference range. Amylase isoenzyme electrophoresis of serum and urine predominantly showed salivary-type total amylase (97.1% and 92.7%, respectively) (Fig. 2). Unfortunately, amylase isoenzyme electrophoresis of pleural effusion was not performed.
On the basis of these findings, the patient was finally diagnosed as having salivary-type amylase-producing mediastinal plasmacytoma with myelomatous pleural effusion. He received two courses of induction chemotherapy consisting of intravenous thalidomide, cyclophosphamide, and dexamethasone. For the management of the mediastinal mass and myelomatous pleural effusion, radiotherapy was administered. After chemotherapy with radiotherapy, urine amylase (2,042.6 IU/L) and lambda light chain (504 mg/L) levels were markedly decreased.
Extramedullary plasmacytoma in multiple myeloma is associated with aggressive disease, leading to shorter overall survival and progression-free survival [2]. Because the human salivary amylase gene (AMY1) is located in chromosome 1p21 [3, 4], the chromosome 1p deletion in our patient resulted in the structural deletion of chromosome 1p21, and such structural alteration might cause an uncontrolled production of amylase by plasma cells, leading to an elevated amylase level. Duplication of all or part of the 1q chromosome and whole arm translocation of 1q in multiple myeloma are associated with tumor progression and advanced disease [5, 6].
Our patient had high amylase levels in the serum, urine, and pleural fluid. After induction chemotherapy and radiotherapy for mediastinal plasmacytoma and myelomatous pleural effusion, amylase levels in the serum and urine were markedly decreased. This finding suggests that the neoplastic plasma cells directly produced salivary-type amylase in this case. This evidence supports the hypothesis that salivary-type amylase levels in serum, urine, and other fluids are indicative of myeloma disease progression and treatment response [7]. A recent research revealed that patients with amylase-producing myeloma commonly present with salivary-type amylase isoenzyme production, high tumor mass, extensive extramedullary spread, extensive bone destruction, and poor prognosis; therefore, in these patients, a simple test such as serum amylase level may represent a reliable disease activity index and provide additional prognostic information [7].
To our knowledge, salivary-type amylase-producing multiple myeloma presenting with mediastinal plasmacytoma and myelomatous pleural effusion as seen in our patient is extremely rare. Although the precise mechanism of development of hyperamylasemia in myeloma is not well understood, chromosome 1p deletion in this patient can result in a functional change in the AMY1 gene located on chromosome 1p21. In patients with salivary-type amylase-producing multiple myeloma, a simple test such as estimating serum amylase levels may provide reliable disease activity and prognostic information.
References
1. Masood A, Hudhud KH, Hegazi A, Syed G. Mediastinal plasmacytoma with multiple myeloma presenting as a diagnostic dilemma. Cases J. 2008; 1:116. PMID: 18717993.

2. Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010; 21:325–330. PMID: 19633044.

3. Delannoy A, Hamels J, Mecucci C, Fally P, Wallef G, de Fooz C, et al. Amylase-producing IgD-type multiple myeloma. J Intern Med. 1992; 232:457–460. PMID: 1280672.

4. Ohtsuki T, Yawata Y, Wada H, Sugihara T, Mori M, Namba M. Two human myeloma cell lines, amylase-producing KMS-12-PE and amylase-non-producing KMS-12-BM, were established from a patient, having the same chromosome marker, t(11;14)(q13;q32). Br J Haematol. 1989; 73:199–204. PMID: 2479409.

5. Lai JL, Zandecki M, Mary JY, Bernardi F, Izydorczyk V, Flactif M, et al. Improved cytogenetics in multiple myeloma: a study of 151 patients including 117 patients at diagnosis. Blood. 1995; 85:2490–2497. PMID: 7537117.

6. Sawyer JR, Tricot G, Mattox S, Jagannath S, Barlogie B. Jumping translocations of chromosome 1q in multiple myeloma: evidence for a mechanism involving decondensation of pericentromeric heterochromatin. Blood. 1998; 91:1732–1741. PMID: 9473240.

7. Pinelli M, Bindi M, Rosada J, Scatena P, Castiglioni M. Amylase: a disease activity index in multiple myeloma. Leuk Lymphoma. 2006; 47:151–154. PMID: 16321841.

Fig. 1
Histologic examination of the pleural fluid (A), mediastinal mass (B and C), and bone marrow (D, E, and F). (A) Cytospin analysis of pleural fluid shows increased plasma cells with eccentric nuclei and abundant basophilic cytoplasm, consistent with myelomatous pleural effusion (Wright-Giemsa stain, ×200). (B) The histologic examination of mediastinal plasmacytoma reveals diffuse infiltration of mature plasma cells (hematoxylin and eosin stain, ×200). (C) Immunohistochemical staining of mediastinal plasmacytoma shows CD138 positivity (×1,000). (D) Bone marrow aspiration shows moderate proliferation of neoplastic plasma cells, consistent with plasma cell myeloma (Wright-Giemsa stain, ×1,000). (E and F) Immunohistochemical staining of bone marrow shows monoclonal proliferation of lambda-restricted plasma cells (kappa [E] and lambda [F] stains, ×200).
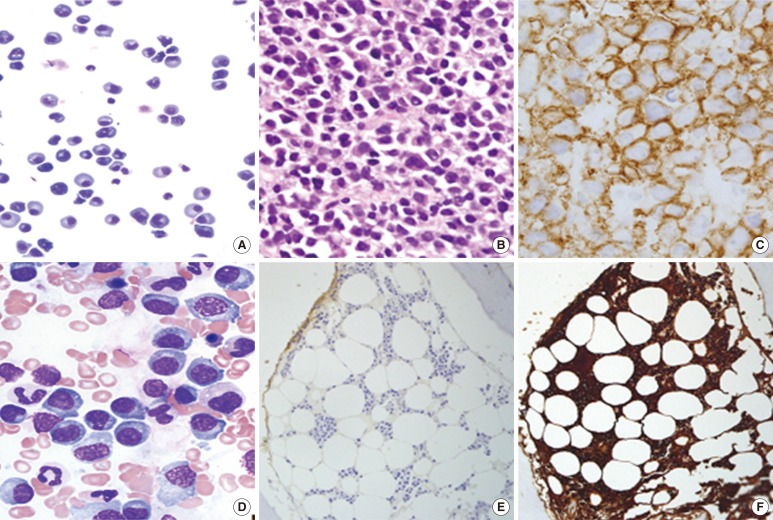
Fig. 2
Amylase isoenzyme electrophoreses of serum and urine shows that the level of the salivary-type isoenzyme is the highest, consistent with salivary-type amylase-producing myeloma.
Abbreviations: S1, salivary-type isoenzyme 1; S2, salivary-type isoenzyme 2; S3, salivary-type isoenzyme 3; P1, pancreatic-type isoenzyme 1.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download