This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Measurement of the ABO antibody (Ab) titer is important in ABO-incompatible transplantation. However, to the best of our knowledge, no standard protocol or external survey program to measure the ABO Ab titer has been established in Korea. We investigated the current status of ABO Ab titer measurements at various laboratories in Korea and the impact of the protocol provided to reduce interlaboratory variations in the methods and results of ABO Ab titers.
Methods
The Korean external quality assessment of blood bank laboratories sent external survey samples with a questionnaire to 68 laboratories across Korea for the measurement of ABO Ab titers in May 2012. After 6 months, a second set of survey samples were sent with a standard protocol to 53 of the previously surveyed laboratories. The protocol recommended incubation at room temperature only and use of the indirect antihuman globulin method for the tube test as well as and the column agglutination test (CAT).
Results
Several interlaboratory variations were observed in the results, technical procedures, and methods selected for measurement. We found that 80.4% laboratories hoped to change their protocol to the provisional one. Additionally, CAT showed significantly lower variation among laboratories (P=0.006) than the tube test.
Conclusions
Our study provides baseline data regarding the current status of ABO Ab titer measurement in Korea. The standard protocol and external survey were helpful to standardize the technical procedures and select methods for ABO Ab titer measurement.
Go to :

Keywords: ABO blood group system, Antibody, Titer, Standard
INTRODUCTION
ABO-incompatible renal transplantations have become increasingly common because of shortage of ABO-compatible donors [
1,
2]. Accurate measurement of the ABO antibody (Ab) titer is important for successful ABO-incompatible transplantations and is mainly determined by the tube test involving observation of agglutinated red blood cells (RBC) with different incubation temperatures, including a phase at ambient room temperature, followed by one at 37℃ and conversion to the antihuman globulin phase [
3]. In addition to reports on large interlaboratory variation in the results of ABO Ab titer measurements [
4,
5,
6], a reduction in this variation has been reported by using a standard protocol or an external survey. However, to the best of our knowledge, neither a standard protocol nor an external survey program for measurement of the ABO Ab titer has been established in Korea.
The aims of the study were to investigate 1) the current status of ABO Ab titer measurements at various laboratories in Korea, and 2) the impact of the provisional standard protocol on interlaboratory variations in the selection of techniques and results of ABO Ab titer measurements.
Go to :

METHODS
The Korean external quality assessment (KEQA) for blood bank laboratories sent fresh frozen plasma (FFP) of blood group O as external survey samples with a questionnaire to 68 laboratories across Korea for the measurement of anti-A and anti-B titers, in May 2012. The samples were prepared as follows: 2 units of thawed, blood group O FFP were mixed well and 3 mL of this FFP was sent as samples to the laboratories. The questionnaire consisted of 19 questions regarding the technical procedures used, the RBC suspension, the specimen, treatment with dithiothreitol (DTT), and reading the end-point, etc. for each technique. After 6 months, a second set of survey samples from FFP of blood groups O and B were sent with the final report from the first external survey as well as a provisional, standard protocol (defined as standard protocol in the study) to 53 of the laboratories, which participated in the first survey.
The techniques for measurement of the ABO Ab titer were defined as IS for procedures with immediate spin without incubation, RT for procedures with room temperature incubation, 37℃ for procedures with 37℃ incubation, and AHG for procedures using the indirect antihuman globulin method for the tube test as well as the column agglutination test (CAT), irrespective of the use of DTT. The protocol allowed the participant laboratory to select one or more methods and techniques.
The standard protocol was written after the review of literature [
4,
5,
7,
8,
9]. And the Summary of Uniform Procedure of Anti-A titer in ABT from the College of American Pathologists (CAP), results of the first survey in our study (
Table 1), and review by experts were also included in the protocol. A standard protocol was recommended for aspects such as the technical procedure, the type of sample [
7,
8], method of treatment with DTT, use of 0.9% normal saline as diluents [
4,
5,
7,
8], method of dilution of the sample [
9], incubation [
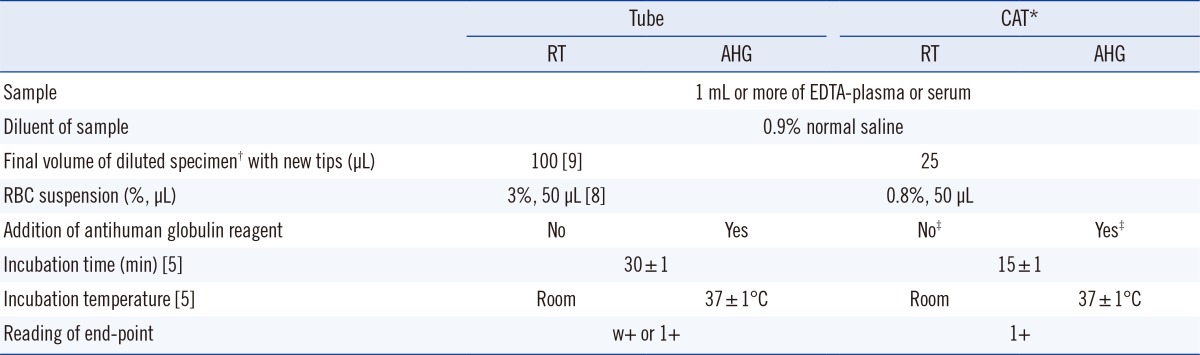
5], and reading of the end point for the tube test. The standard protocol for CAT was as per the manufacturer's instructions. The standard protocol recommended RT conditions only and AHG technique for the tube test as well as CAT, irrespective of whether DTT was used. A summary of the procedures is shown in
Table 2.
Table 1
Number (%) of laboratories and their responses to items in the questionnaire in the first survey
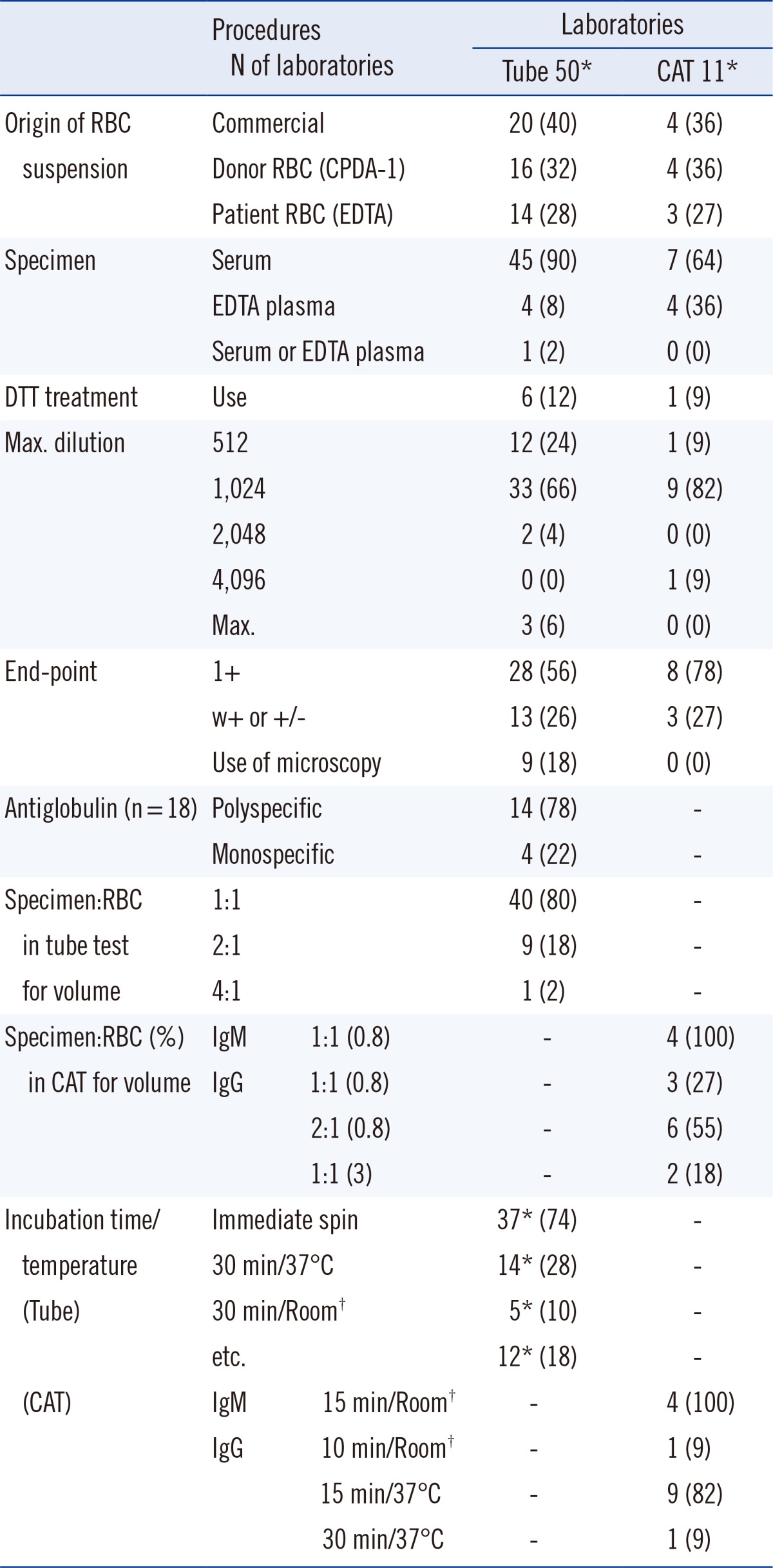

Table 2
Summary of the recommended protocol for measuring the ABO antibody titer in the second survey

The acceptable range of the titer results was defined as one dilution on either side of the most common result plus an additional dilution in the direction of the log, mean value among 10 or more responders. The number of titer steps was defined as the frequency of dilution.
All the statistical analyses were performed by using a software program (SPSS for Windows v. 12.0; SPSS, Chicago, IL, USA). The rates at which results from various laboratories were found to be within the acceptable range of the ABO Ab titer were analyzed statistically by using the Pearson Chi-square test and/or Fisher's exact test with 2-sided exact significance. Differences in the number of titer steps between techniques and the survey were analyzed statistically by using the Student t-test and the paired t-test, respectively. A P value less than 0.05 was considered statistically significant.
Go to :

RESULTS
1. Current status of ABO Ab titer measurement at laboratories in Korea
The response rates were 77.9% (53/68) for the first survey and 86.8% (46/53) for the second survey.
As shown in
Table 1, several interlaboratory variations were found in the procedures used, including those with respect to the dilution, reading of the end point, incubation, sample, and reagents. Most of the laboratories used either serum as the sample (90%), polyspecific AHG (78%), 1:1 (v/v) ratio of specimen to RBC (80%), or immediate spin condition for incubation (74%) in the tube test. Additionally, 18% (9/53) of the laboratories used microscopy when reading the end-points with the tube test (
Table 1).
Of the laboratories that responded to the first survey, 36 were part of university hospitals, while 17 were affiliated to tertiary or secondary hospitals. Among these, only 28 laboratories reported to have conducted ABO Ab titer testing at least once in the past year (2011). Fifty laboratories (excluding duplicates) reported using the tube test with IS (37), RT (8), 37℃ (5), and AHG (18), while 11 laboratories (excluding duplicates) reported using the CAT-based gel card test with RT (4) and AHG (11) (
Table 3). Thirty laboratories used only one, 16 used two, while 7 used three techniques. Of the remaining 25 laboratories where any ABO Ab titer measurements had not been conducted in 2011, 19 reported to have previously used only the tube-test with IS. In all, only 24 (45%) laboratories used the AHG method in the tube test or CAT for IgG type of ABO Ab testing.
Table 3
Number of laboratories, listed as per techniques used, for ABO Ab titer measurement in both surveys
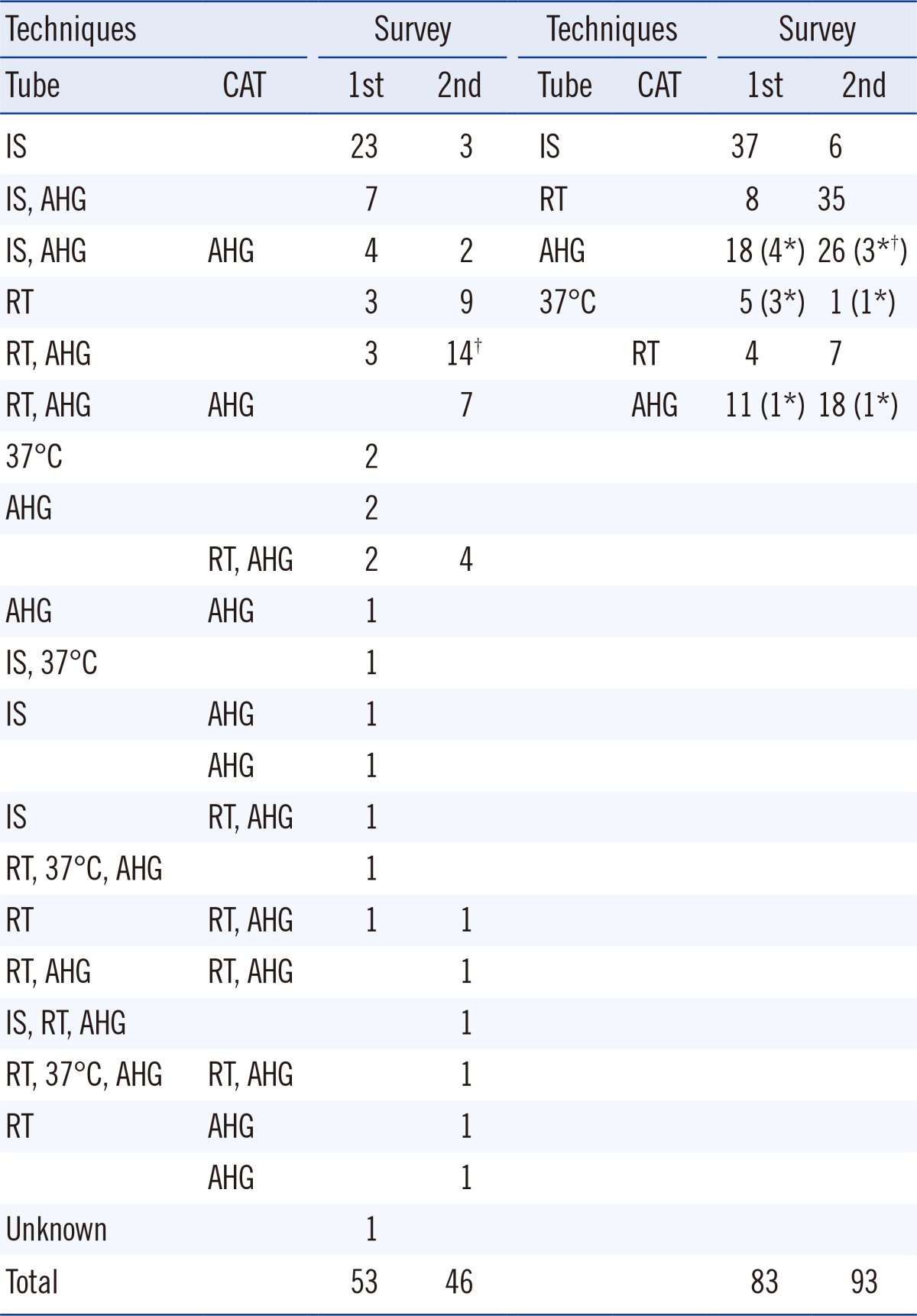

2. Effects of the provisional standard protocol on interlaboratory variation
In the second survey, 80.4% (37/46) laboratories answered that they would change their protocol to the standard protocol of measuring the ABO Ab titer. After receiving the standard protocol, many laboratories chose to use the recommended techniques from the standard protocol. The proportion of laboratories (number of participants that used the tube-test/number of total participants) increased from 15.1% (8/53) to 76.1% (35/46) for RT and from 34.0% (18/53) to 56.5% (26/46) for AHG in the second survey. The proportion of laboratories (number of participants that used CAT/number of total participants) increased from 7.5% (4/53) to 15.2% (7/46) for RT and from 20.8% (11/53) to 37.0% (17/46) for AHG in the second survey (
Table 3). None of the laboratories used microscopy to read the end-point for the tube test in the second survey.
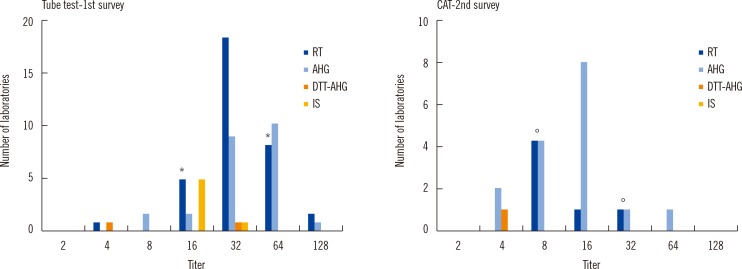
The distribution of titer results for anti-A in
Fig. 1 and anti-B in
Fig. 2 for blood group O and that for anti-A in
Fig. 3 for blood group B are shown, grouped as per the technique used. The maximum differences of titer step (range of titer) between laboratories for Tube-RT including Tube-IS and Tube-AHG were six (4-256) and five (16-512) for the first survey and five (4-128) and five (8-256) for the second survey, respectively. The differences of titer step (range of titer) for CAT-RT and CAT-AHG were one (16-32) and zero (128) for the first survey and two (8-32) and three (16-128) for the second survey, respectively. Difference in the average of titer steps in CAT (1.5) was significantly lower than that for the tube test (5.25,
P=0.006). This finding indicates that CAT has significantly less interlaboratory variation than the tube test. However, there was no significant difference in the number of titer steps between the first (3.0) and second (3.75) survey.
 | Fig. 1
Number of laboratories with distribution of the anti-A titers as per method and survey results in blood group O. Number of laboratories reading '1+' as the only end point; The asterisk (*) and empty circle (°) mean the acceptable range of the titers, which was defined as one dilution either side of the most common response plus an additional dilution in the direction of the log mean response among 10 or more responders, for RT or IS and AHG, respectively. There were no significant differences in the rate at which laboratory results were within acceptable range using the tube test between both surveys; the rates were 77.8% (33/45) and 87.8% (36/41) for Tube-RT including Tube-IS (P=0.110) and 78.6% (11/14) and 92.0% (23/25) for Tube-AHG (P=0.329).
Abbreviations: IS, procedures with immediate spin; RT, procedures with room temperature incubation; 37℃, procedures with 37℃ incubation; AHG, procedures with indirect antihuman globulin test; CAT, column agglutination test; DTT-37℃, procedure with 37℃ incubationin dithiothreitol treated sample; DTT-AHG, procedures with indirect antihuman globulin test in dithiothreitol treated sample.

|
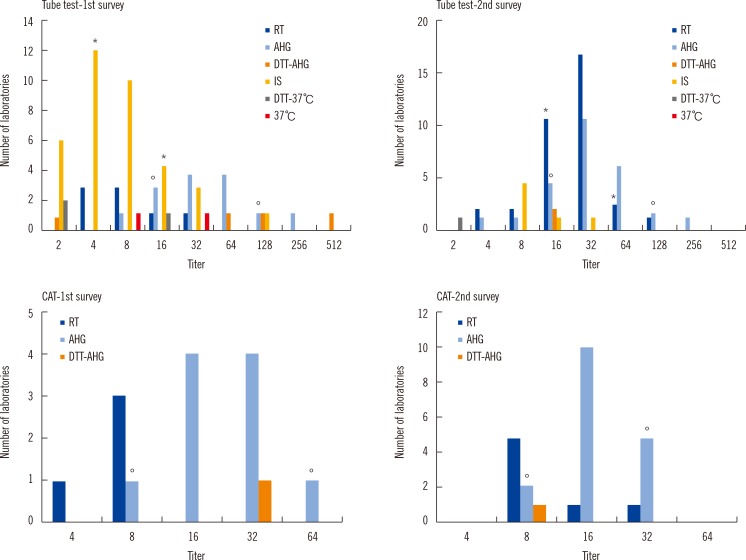 | Fig. 2
Number of laboratories with distribution of the anti-B titer as per method and survey results, in blood group O. There were no significant differences in the rate at which laboratory results were within acceptable range using the tube test between both surveys; the rates were 88.9% (40/45) and 78.0% (32/41) for Tube-RT including Tube-IS (P=0.244) and 85.7% (12/14) and 88.0% (22/25) for Tube-AHG (P=1.000). Number of laboratories reading '1+' as the only end point; Asterisk (*), empty circle (°).
Abbreviations: IS, procedures with immediate spin; RT, procedures with room temperature incubation; 37℃, procedures with 37℃ incubation; AHG, procedures with indirect antihuman globulin test; CAT, column agglutination test; DTT-37℃, procedure with 37℃ incubationin dithiothreitol treated sample; DTT-AHG, procedures with indirect antihuman globulin test in dithiothreitol treated sample.

|
 | Fig. 3
Number of laboratories with distribution of the anti-B titer as per method in the second survey, in blood group A. Number of laboratories reading '1+' as the only end point; Asterisk (*), empty circle (°).
Abbreviations: IS, procedures with immediate spin; RT, procedures with room temperature incubation; 37℃, procedures with 37℃ incubation; AHG, procedures with indirect antihuman globulin test; CAT, column agglutination test; DTT-37℃, procedure with 37℃ incubationin dithiothreitol treated sample; DTT-AHG, procedures with indirect antihuman globulin test in dithiothreitol treated sample.

|
There were no significant differences in the rate at which laboratory results were within acceptable range using the tube test between both surveys: the rates of anti-A of blood group O were 77.8% (33/45) and 87.8% (36/41) for Tube-RT including Tube-IS (P=0.110) and 78.6% (11/14) and 92.0% (23/25) for Tube-AHG (P=0.329); the rates for anti-B of blood group O were 88.9% (40/45) and 78.0% (32/41) for Tube-RT including Tube-IS (P=0.244) and 85.7% (12/14) and 88.0% (22/25) for Tube-AHG (P=1.000).
Go to :

DISCUSSION
In previous studies, we found interlaboratory variations in IgM-type ABO Ab titer as large as 16-fold [
6], 32-fold [
4], and 32-fold using Tube-RT, while in IgG-type ABO Ab titer variations were up to 32-fold [
6], 256-fold [
4], and 64-fold using Tube-AHG techniques. The study from three European centers reported that there was a six-titer-step difference in the tube test among institutions. The interlaboratory variation for the IgG tends to be higher than that for the IgM ABO Ab titer. This may be related to the more complicated techniques involved in IgG ABO Ab titer measurement than that used for IgM. Differences in the ABO Ab titer have been reported not only between laboratories but also between techniques in the same laboratory [
10]. Therefore, a standard protocol is necessary to reduce inter- and intralaboratory titer variations.
The standard protocol recommends EDTA-plasma or serum to be used as the sample since serum is widely used [
8] while EDTA in plasma can prevent complement activation and hemolysis during incubation at 37℃ [
7]. Therefore, if a laboratory uses the AHG technique for ABO Ab titer measurement, then EDTA-plasma would be the specimen of choice. For diluting the sample, 0.9% normal saline without any potentiator is the recommended diluent, since any form of enhancement may result in false elevation of titers [
9]. Either 2 drops of 2% RBC suspension or 1 drop of 3% RBC suspension can be used [
8,
9]. In our study, we selected 1 drop of 3% RBC suspension, as 40% of laboratories in our study used the 3% commercial RBC suspension for the measurement of the ABO Ab titer.
CAP recommends w+ or the trace end-point instead of 1+ reactivity to reduce interlaboratory variability as the difference between 1+ and 2+ may be less discernible than that between w+ and 1+ [
5]. Although our study could not analyze the effect of w+ reactivity owing to the limited responders with the w+ end-point, a similar tendency could not be found in our study. Since other reports recommended the 1+ end-point, except for CAP, our protocol recommended the w+ or 1+ end-point as per the laboratory, without the use of microscopy when using the tube test [
8]. After distribution of the standard protocol, 18% (9/53) of the laboratories that used microscopy in the first survey changed their protocols to macroscopic observations.
Before the distribution of the standard protocol, 55% of the laboratories in Korea used only Tube-IS, Tube-RT, and Tube-37℃ methods. Although both IgM and IgG can agglutinate RBC at room temperature or cooler [
9], these techniques primarily detect IgM, while IgG cannot be accurately measured. However, AHG titer values are known to be critical for the clinical management of ABO-incompatible kidney transplantation, and IS or RT titers offer no additional data to influence clinical decisions [
11]. IgG is the major isotype for anti-A and anti-B in blood group O, while IgM is the predominant isotype found in group A and group B individuals [
9]. In our study, 80% of laboratories showed willingness to change their protocols for ABO Ab titer measurement according to the standard protocol, and there was a 70-80% increase in the number of laboratories using Tube-AHG or CAT-AHG in the second survey as compared to the first survey. The standard protocol thus contributed to a change in the technique used by local laboratories for ABO Ab titer measurements to detect IgG.
Although the Tube-IS is simpler and faster than Tube-RT and 70% (37/53) of the laboratories in our study used it, our standard protocol chose Tube-RT for detecting the IgM type of ABO Ab to conform to CAP [
5], which recommends 30±1 min as the incubation time for Tube-RT.
A study from 3 European centers showed that CAT significantly decreased intercenter variation as compared to the tube test [
12]. In our study, CAT also showed a significantly lower difference in the reported titer range than the tube test. Moreover, CAT is known to reduce the turnaround time of the ABO AB titer by 50% [
3].
However, the reagents for CAT are very expensive under the domestic health insurance system; thus the routine use of the CAT technique is not feasible in Korea. The practical difficulties of using CAT for routine measurement of the ABO Ab titer should be discussed in further detail to reduce interlaboratory variation in results.
In conclusion, we found interlaboratory variations in the results, methods, and technical procedures of measurement of the ABO Ab titer in Korea. Our study provides baseline data regarding the current status of ABO Ab titer measurement in Korea, and the standard protocol was helpful to standardize the technical procedures and selection of methods for ABO Ab titer measurement. Although the standard protocol could not significantly reduce interlaboratory variation in the results of the second survey, a periodically conducted external survey could help in continued improvement of the results of ABO Ab titer measurement.
Go to :

Acknowledgments
This work was supported by the KAQACL Research Fund of 2012-2. We thank all the 53 participating laboratories and their staff for their cooperation in this study.
Go to :

Notes
Go to :

References
1. Alexandre GP, Squifflet JP, de Bruyere M, Latinne D, Moriau M, Ikabu N, et al. Splenectomy as a prerequisite for successful human ABO-incompatible renal-transplantation. Transplant Proc. 1985; 17:138–143.
2. Chang CL, Jeong JH, Kim JP, Lee DR, Kong JM, Kim BC. A single center experience of ABO incompatible kidney transplantation. J Korean Soc Transplant. 2012; 26:261–268.

3. Tobian AA, Shirey RS, King KE. ABO antibody titer monitoring for incompatible renal transplantation. Transfusion. 2011; 51:454–457. PMID:
21388388.

4. Kobayashi T, Saito K. A series of surveys on assay for anti-A/B antibody by Japanese ABO-incompatible Transplantation Committee. Xenotransplantation. 2006; 13:136–140. PMID:
16623808.

5. AuBuchon JP, de Wildt-Eggen J, Dumont LJ. Reducing the variation in performance of antibody titrations. Vox Sang. 2008; 95:57–65. PMID:
18479347.

6. Lee EY, Kim S, Kim HO, Kwon SW, Kim DW, Han KS. Survey analysis of ABO antibody titration at four university hospitals in Korea. Korean J Blood Transfus. 2011; 22:24–30.
7. Roback JD, Grossman BJ, Harris T, Hillyer CD, editors. Technical manual. 17th ed. Bethesda: American Association of Blood Banks;2011. p. 369.
8. Roback JD, Grossman BJ, Harris T, Hillyer CD, editors. Technical manual. 17th ed. Bethesda: American Association of Blood Banks;2011. p. 907–910.
9. Roback JD, Grossman BJ, Harris T, Hillyer CD, editors. Technical manual. 17th ed. Bethesda: American Association of Blood Banks;2011. p. 935–937.
10. Kang MG, Lee SJ, Oh JS, Lim YA. Comparison of ABO isoagglutinin titers by different tube hemagglutination techniques. Korean J Blood Transfus. 2009; 20:227–234.
11. Tobian AA, Shirey RS, Montgomery RA, Ness PM, King KE. The critical role of plasmapheresis in ABO-incompatible renal transplantation. Transfusion. 2008; 48:2453–2460. PMID:
18657072.

12. Kumlien G, Wilpert J, Säfwenberg J, Tydén G. Comparing the tube and gel techniques for ABO antibody titration, as performed in three European centers. Transplantation. 2007; 84(12 Suppl):S17–S19. PMID:
18162980.

Go to :











 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download