This article has been
cited by other articles in ScienceCentral.
Autosomal trisomy as a sole cytogenetic change has been described in several hematologic malignancy cases, but the number of reports on the association between specific morphologies and individual structural cytogenetic abnormalities are few [
1]. Trisomy 8 is the most common abnormality in myeloproliferative neoplasms (MPN), MDS, MDS/MPN and AML, and trisomy 4, 9, 11, 13, and 21 have been reported to be associated with myeloid disorders [
1,
2]. Here, we describe three patients with AML and show that trisomy 6 is the sole clonal cytogenetic bone marrow abnormality. The patients were adults without previous hematologic disorders, and were initially diagnosed with AML M1, M2, and M4, according to the French-American-British (FAB) classification. These patients received chemotherapy with or without allogenic peripheral blood stem cell transplantation (PBSCT), and showed variable clinical outcomes.
The first case of sole trisomy 6 abnormality was reported in a patient with aplastic anemia (AA) [
2]. The other reports showed that trisomy 6 was associated with hypoplastic bone marrow, dyserythropoiesis and AML prior to hypocellular marrow with MDS [
3,
4,
5]. To establish a relationship between the clonality of AML and a single trisomy 6 abnormality, we searched PubMed (
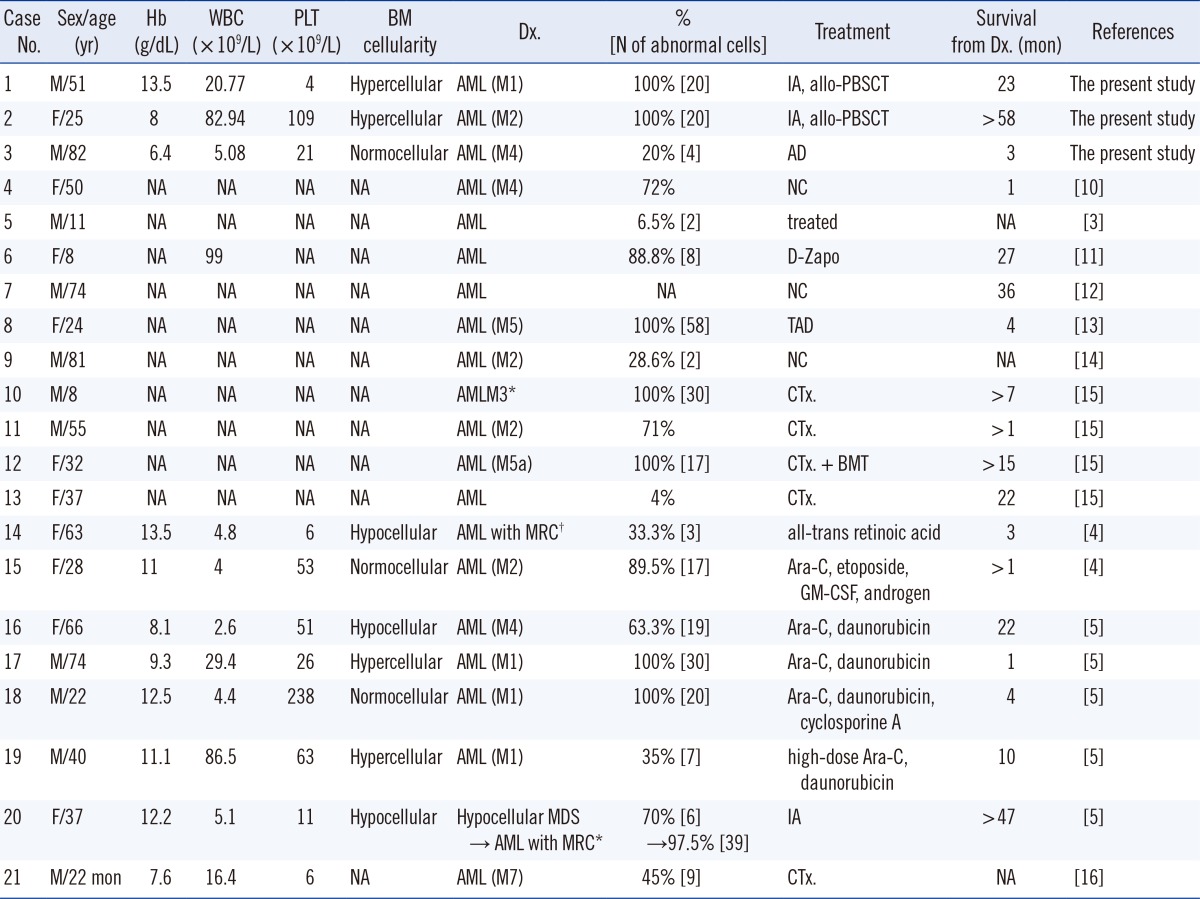
http://www.ncbi.nlm.nih.gov/pubmed) with combinations of the search terms "AML" and "trisomy 6". Subsequently, we identified ten reports describing 18 cases of AML presenting with trisomy 6 as the sole karyotypic abnormality. The laboratory findings and clinical features, including those of the three cases reported here, and those of previously reported cases are summarized in
Table 1. Including our three cases, there were 10 female and 11 male patients, including 3 children and 1 infant. The mean age was 41.4 yr, with a range of 22 months to 82 yr. Their bone marrow (BM) cellularity was variable, but it showed a hypo- to normocellular tendency. Although some cases were reported without the results of a complete blood count (CBC), we found six cases of cytopenia in a single cell line (five cases of thrombocytopenia and one case of anemia), three cases of cytopenia in two cell lines (both anemia and thrombocytopenia), and one case of pancytopenia. Leukocytosis was found in six of the 11 cases with CBC results. There were four cases each of M1 and M2, three cases of M4, two cases of M5, and one case of M7 diagnosed according to FAB criteria; four cases of the 21 were diagnosed as having AML without additional categorization. Case number (No.) 10 was suspicious for AML-M3, but no rearrangement of t(15;17)(q22;q12) was identified, so it was not presumed to be AML-M3. Case No.14 was reported as relapsed AML from refractory anemia with excess of blasts in transformation (RAEB-t), but we reclassified the case as AML with myelodysplasia-related changes according to the 2008 WHO classification. Case No. 20 was similarly reclassified as AML with myelodysplasia-related changes owing to MDS, according to the 2008 WHO classification. This patient initially showed hypocellular BM with mild dysplastic changes and 47,XX,+6[14]/46,XX[6], and then experienced transformation into AML 15 months later with an increase in the proportion of trisomy 6 cells and 47,XX, +6[39]/46,XX[1]. Mohamed et al. [
5] mentioned that the increase in blasts was associated with an increase in the proportion of trisomy 6 cells. In our three cases, case No. 1; 81% of blasts with 47,XY,+6[20], No. 2; 60% of blasts with 47,XX,+6[20] and No. 3; 50% of blasts with 47,XY,+6[4]/46,XY[16] showed that there were no direct correlations between the number of blasts and the percentage of abnormal metaphases. Most patients with this abnormality received chemotherapy. Bone marrow transplantation (BMT) or PBSCT was performed in three of the cases. The survival from the time of diagnosis was variable and ranged from one month to longer than 58 months.
We reported three Korean patients with AML with a particular genetic abnormality, showing the presence of trisomy 6 as the sole clonal cytogenetic abnormality of BM cells and reviewed other cases of AML with trisomy 6 in the literature. Although other studies and literature reviews that have attempted to establish the clinicopathologic and clinicohematologic characteristics of AML with sole trisomies 4, 8, 11, and 21 [
6,
7,
8,
9] were found, we could not identify any correlation between morphology or prognosis and trisomy 6. In view of the literature, we suggest that trisomy 6 is a nonrandom clonal cytogenetic marker of AML, and it may sometimes be the result of transformation from other myeloid disorders. Further studies to establish the clonality of AML with sole trisomy 6 are needed.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download