CML is a myeloproliferative disease characterized by the Philadelphia (Ph) chromosome, in which the oncogenic BCR-ABL1 fusion gene encodes a constitutively active tyrosine kinase. First-line treatment using the BCR-ABL tyrosine kinase inhibitor (TKI) imatinib has significantly changed the disease course of CML [1]. However, some patients develop resistance to this agent, largely due to point mutations within the ABL1 kinase domain (KD) [2, 3]. Resistance to imatinib may be overcome by treatment with second-line TKIs, including dasatinib, nilotinib, and bosutinib, which are active against most mutations; however, some mutations bring about resistance to these second-line drugs as well [4, 5, 6]. Therefore, a mutation analysis is recommended when choosing a second-line TKI [7]. We describe a CML patient who rapidly progressed to blast crisis following the sequential acquisition of ABL1 KD mutations with a complex karyotype.
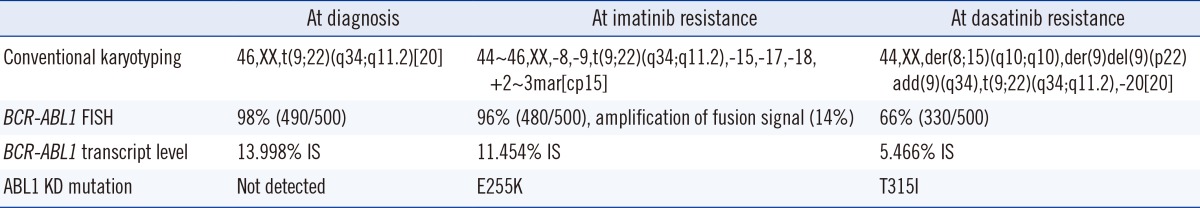
A 45-yr-old female patient was admitted to National Cancer Center (Goyang, Korea) in December 2012 for marked leukocytosis and splenomegaly. Her peripheral blood and bone marrow (BM) findings were consistent with CML in the chronic phase. Conventional karyotyping indicated the presence of a Ph chromosome and no other abnormalities. FISH using a BCR/ABL1 dual-fusion triple color translocation probe showed 98% BCR-ABL1 gene fusion with a 13.998% international scale (IS) increase in BCR-ABL1 transcript levels. Further study with direct sequencing of the ABL1 KD detected no point mutations. The patient's Sokal risk score [8] was 0.8 (intermediate) and her Hasford risk score [9] was 433.6 (low). The patient was started on an imatinib dosage of 400 mg/day. Complete hematologic response (CHR) was achieved within two weeks, and the BCR-ABL1 transcript level after one month was 1.993% IS.
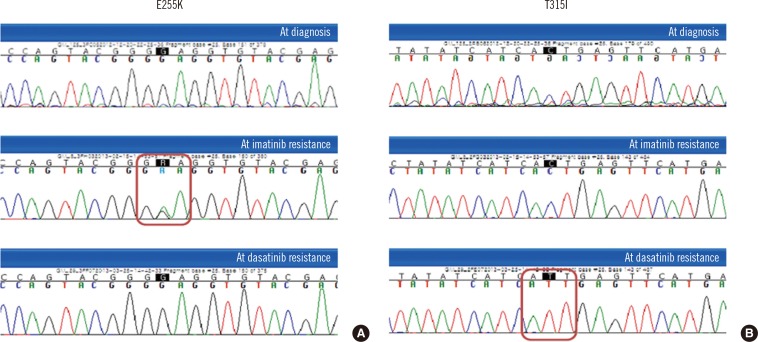
On day 53 of treatment, the patient visited the emergency room with fever and myalgia. Her white blood cell (WBC) count had increased to 256×109/L with 89% blasts. Flow cytometric analysis showed that 98.3% of the blasts were positive for CD34, 84.0% for terminal deoxynucleotidyl transferase (TdT), 99.4% for CD19, 47.3% for CD20, 99.7% for HLA-DR, and 49.5% for CD33. They were negative for CD10 and other T-cell and myeloid lineage markers; these findings were consistent with those for B lymphoid blasts. Imatinib was discontinued and replaced with dasatinib (140 mg/day). A BM examination three days later showed a hypocellular marrow with an increased number of blasts. Conventional karyotyping showed complex numerical and structural abnormalities: 44~46,XX,-8,-9,t(9;22)(q34;q11.2),-15,-17,-18,+2~3mar[cp15]. FISH showed BCR-ABL1 translocation of 96%, with 14% showing a three-fusion signal of amplification, a finding known to be associated with imatinib resistance [3, 10]. The BCR-ABL1 transcript level increased to 11.454% IS. E255K, a dasatinib-sensitive mutant, was detected by direct sequencing (Fig. 1A) [6].
One week after starting dasatinib, the patient achieved CHR, but on day 12, her WBC count began to increase and blasts reappeared. Owing to a resistance to dasatinib, she was started on intensive cytotoxic induction chemotherapy. A BM examination one month later showed a 5.466% IS increase in blasts with BCR-ABL1 transcript levels. Conventional cytogenetic analysis showed clonal evolution: 44,XX,der(8;15)(q10;q10),der(9)del(9)(p22)add(9)(q34),t(9;22)(q34;q11.2),-20[20]. FISH results showed BCR-ABL1 translocation of 66%; however, amplification of the fusion signal was not observed. Moreover, mutation analysis, in this case, showed the presence of T315I and the previously identified E255K was not detected (Fig. 1B). The patient received a second cycle of chemotherapy, but her general condition worsened and she developed multiple organ failure.
We describe here the case of a CML patient who rapidly developed imatinib resistance within two months and dasatinib resistance within two weeks, each instance accompanied by a corresponding ABL1 KD mutation. The laboratory findings at the time of diagnosis are summarized in Table 1. The presence and evolution of mutations during TKI treatment need to be considered for optimal disease control. We performed mutational screening by direct sequencing, the method currently recommended for ABL1 KD mutation analysis [11]. However, this method cannot detect subclones present in less than 10-20% of the BCR-ABL1 cell pool. Thus, subclones harboring mutations may have been present but could not be detected, possibly having undergone clonal expansion after the depletion of subclones sensitive to TKIs [12, 13, 14]. However, a more sensitive method of genomic analysis may reveal the presence of these low-level mutations and provide critical information for selecting subsequent therapy and predicting responses.
References
1. Hochhaus A, O'Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009; 23:1054–1061. PMID: 19282833.

2. von Bubnoff N, Schneller F, Peschel C, Duyster J. BCR-ABL gene mutations in relation to clinical resistance of Philadelphia-chromosome-positive leukaemia to STI571: a prospective study. Lancet. 2002; 359:487–491. PMID: 11853795.

3. Hochhaus A, Kreil S, Corbin AS, La Rosée P, Müller MC, Lahaye T, et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia. 2002; 16:2190–2196.

4. Soverini S, Colarossi S, Gnani A, Castagnetti F, Rosti G, Bosi C, et al. Resistance to dasatinib in Philadelphia-positive leukemia patients and the presence or the selection of mutations at residues 315 and 317 in the BCR-ABL kinase domain. Haematologica. 2007; 92:401–404. PMID: 17339191.

5. Hughes T, Saglio G, Branford S, Soverini S, Kim DW, Müller MC, et al. Impact of baseline BCR-ABL mutations on response to nilotinib in patients with chronic myeloid leukemia in chronic phase. J Clin Oncol. 2009; 27:4204–4210.

6. Müller MC, Cortes JE, Kim DW, Druker BJ, Erben P, Pasquini R, et al. Dasatinib treatment of chronic-phase chronic myeloid leukemia: analysis of responses according to preexisting BCR-ABL mutations. Blood. 2009; 114:4944–4953.

7. Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009; 27:6041–6051.

8. Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in "good-risk" chronic granulocytic leukemia. Blood. 1984; 63:789–799. PMID: 6584184.

9. Hehlmann R, Ansari H, Hasford J, Heimpel H, Hossfeld DK, Kolb HJ, et al. German chronic myeloid leukaemia (CML)-Study Group. Comparative analysis of the impact of risk profile and of drug therapy on survival in CML using Sokal's index and a new score. Br J Haematol. 1997; 97:76–85.

10. Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001; 293:876–880.

11. Soverini S, Hochhaus A, Nicolini FE, Gruber F, Lange T, Saglio G, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011; 118:1208–1215.

12. Ernst T, Gruber FX, Pelz-Ackermann O, Maier J, Pfirrmann M, Müller MC, et al. A co-operative evaluation of different methods of detecting BCR-ABL kinase domain mutations in patients with chronic myeloid leukemia on second-line dasatinib or nilotinib therapy after failure of imatinib. Haematologica. 2009; 94:1227–1235.

Fig. 1
The mutational analysis of ABL1 kinase domain with conventional direct sequencing at the time of diagnosis (top row), after development of imatinib resistance (middle row), and after development of dasatinib resistance (bottom row). (A) E255K (c.763G>A) was detected after development of imatinib resistance. (B) T315I (c.944C>T) was first detected after switchover to dasatinib resistance.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download