Abstract
Background
The dramatic increase in use of the IgG test for toxoplasma, rubella, cytomegalovirus (CMV), and herpes simplex virus (HSV) [TORCH] has led to the requirement for a high-efficiency method that can be used in the clinical laboratory. This study aimed to compare the results of BGI-Array ELISA TORCH IgG (BGI-GBI, China) screening method to those of Virion/Serion TORCH IgG ELISA (Virion/Serion, Germany).
Methods
Serum specimens (n=400) submitted for routine IgG testing by Virion/Serion ELISA were also tested using the BGI-Array ELISA method. The agreements of these two kinds of method were analyzed by κ-coefficients calculation.
Results
Following repeat testing, the BGI-Array ELISA TORCH IgG assays demonstrated agreements of 99.5% (398/400 specimens), 98% (392/400 specimens), 99% (396/400 specimens), and 99.5% (398/400 specimens), respectively. The BGI-Array ELISA IgG assays provided results comparable to Virion/Serion ELISA results, with κ-coefficients showing near-perfect agreement for the HSV (κ=0.87), rubella (κ=0.92) and CMV (κ=0.93) and substantial agreement for the toxoplasma (κ=0.80) IgG assays. The use of the BGI-Array ELISA TORCH IgG assays could reduce the turnaround time (1.5 hr vs. 5 hr by Virion/Serion ELISA for 100 specimens) and were easy to use.
TORCH is a medical acronym used to define a set of perinatal infections by Toxoplasma gondii, rubella virus, cytomegalovirus (CMV), and herpes simplex virus (HSV), which can be passed from a pregnant woman to her fetus [1-3]. These infections can lead to severe fetal anomalies or even fetal loss [3]. The severity of these outcomes highlights the importance of the early detection of infectious pathogens. Therefore, serologic screening for these pathogens is considered routine practice in many parts of the world [2]. Conventional methods for the detection of antibodies to TORCH include enzyme immunoassay (EIA), immunofluorescence (IFA), and enzyme-linked fluorescent assay (ELFA). These techniques have been used for years in both diagnostic and screening protocols for TORCH infection, and they have showed reliable performance. However, these methods have certain limitations, such as low throughput and significant hands-on time.
The worldwide requirement for TORCH screening is increasing dramatically, especially in developing countries [3, 4]. Therefore, it is becoming important to improve the efficiency of these tests in a clinical setting. Many studies and manufacturers are focusing on this issue and solutions have been proposed, such as the BioPlex 2200, which was developed by Bio-Rad Laboratories (Hercules, CA, USA) [5-8]. This method is based on the multiplex flow immunoassay (MFI), which uses a liquid suspension array of up to 100 unique microspheres conjugated with different antibodies. Moreover, some reports have proposed an alternative method based on a protein microchip, which combines the pathogens' antigens on a single chip [6, 8, 9]. Both MFI and the protein microchip method can improve throughput and cost-efficiency, as well as reduce the turnaround time (TAT).
The rapidly increasing requirement for TORCH screening encouraged us to introduce a novel BGI-Array ELISA TORCH screening method (BGI-GBI, Beijing, China) in our laboratory, which is a combination of the protein microchip and conventional ELISA methods. In this study, we evaluated clinical samples using the BGI-Array ELISA TORCH IgG immunoassays, and compared the results with those of Virion/Serion ELISA methods.
We collected 400 serum samples from pregnant patients at Suzhou Municipal Hospital in September-November 2012 for TORCH IgG analysis. The blood samples were collected after an overnight fast of 12-14 hr. After clotting, blood was centrifuged at 1,200 g for 10 min to obtain serum for TORCH IgG screening. The serums were analyzed in parallel by using both BGI-Array ELISA and Virion/Serion ELISA methods. Samples within consistent results after initial testing were retested by both Virion/Serion ELISA and BGI-Array ELISA methods with the same samples. Our hospital ethics committee approved this study. The TAT refers to the analysis time, which measures the duration starting at the acceptance the sample and ending at completion the test.
Routine TORCH IgG tests were performed according to the manufacturer's instructions using a commercial ELISA kit (Virion/Serion, Würzburg, Germany). Developing color was quantified on an automatic microliter plate reader (StatFax-3200, Awareness Inc., Palm City, FL, USA). The results were expressed as optical density (OD) at 450/630 nm. The presence of IgG antibodies to TORCH antigens was determined by comparing the absorbance value of serum samples with that of the cutoff value of a standard positive control. The presence of IgG antibodies was classified as positive, negative, or equivocal.
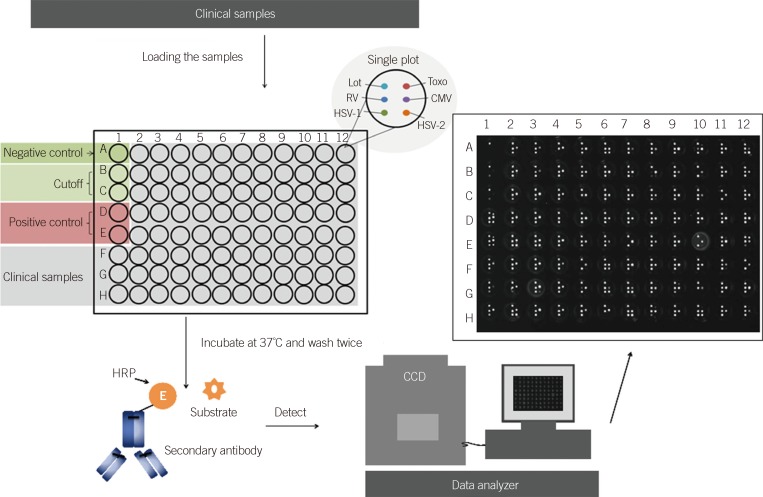
BGI-Array ELISA was performed according to the manufacturer's instructions. Briefly, the serum was diluted at 1:51 with dilution buffer. The negative control, positive control, and cutoff control were set up as shown in Fig. 1. After loading the sample into a 96-well plate, the plate was covered with an adhesive plastic and shaken for 30 min at 37℃. After washing five times, 50 µL of the substrate solution was dispensed per well. Then, the plate was covered again and shaken for 30 min at 37℃. The chemiluminescence reagent was prepared freshly by combining the luminol with hydrogen peroxide at 1:1 and 40 µL was dispensed into each well, avoiding the formation of bubbles. This plate was inserted into the BGI-Array reader (BGI-GBI) for data collection and analysis.
Quality control of the BGI-Array ELISA TORCH assay was performed in accordance with the following conditions. First, the relative light units (RLU) of negative wells should be <200. Second, the RLU of both cutoff wells should be <400, but >200. Lastly, the RLU of positive wells should be >400. If these conditions are not met, the test is assumed to be void and should be repeated. Samples with RLU >400 were defined as positive, and samples with RLU <200 were defined as negative. Samples with RLU less than cutoff but >200 were defined as equivocal.
Nine standard IgG antibodies to Toxoplasma gondii (P1-P9), 20 anti-rubella virus IgG antibodies (R1-R20), 17 anti-HSVI (HI1-HI17), and 9 HSVII (HII1-HII9) antibodies were used for the sensitivity analysis. Ten kinds of negative standard material (N1-N10) were also used. All standard materials were prepared according to the China Food and Drug Administration (CFDA), and the materials were all provided by BGI-GBI.
Statistical analysis was performed by using the Stata12.0 statistical package (Stata Corp., College Station, TX, USA). κ-Coefficients were calculated as a secondary measure of agreement. Agreement results by κ values were categorized as near perfect (0.81-1.0), substantial (0.61-0.8), moderate (0.41-0.6), fair (0.21-0.4), slight (0-0.2), or poor (<0). The difference between the HSV IgG and rubella IgG groups was analyzed by using the Fisher exact test. A P value of <0.05 was considered significant.
The standard materials-9 IgG antibodies to Toxoplasma gondii (P1-P9), 20 anti-rubella virus IgG antibodies (R1-R20), 17 anti-HSVI (HI1-HI17), and 9 HSVII (HII1-HII9) antibodies-gave positive results by the BGI-Array ELISA method for their specific antigens. The negative standard materials (N1-N10) all showed no reaction with the antigen in the BGI-Array. The BGI-Array method has a good specificity and sensitivity with standard materials.
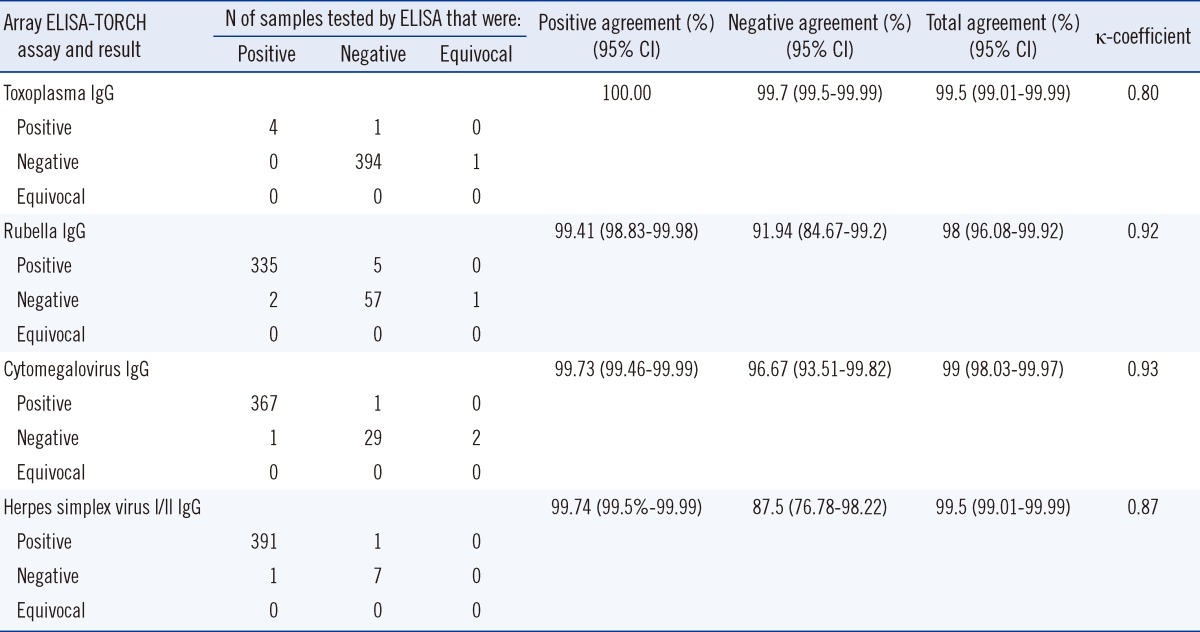
The BGI-Array ELISA TORCH IgG assays demonstrated total agreement of 99.5% (398/400 specimens) for toxoplasma IgG, 98% (392/400 specimens) for rubella IgG, 99% (396/400 specimens) for CMV IgG, and 99.5% (398/400 specimens) for HSV IgG (Table 1). κ-Coefficients showed near-perfect agreement for the HSV (κ=0.87), rubella (κ=0.92) and CMV (κ=0.93) assays and substantial agreement for the toxoplasma (κ=0.80) IgG assays. The prevalence of TORCH pathogens based on the different methods (Virion/Serion ELISA vs. BGI-Array ELISA) was as follows: 1.0% vs. 1.25% for toxoplasma IgG; 84.25% vs. 85% for rubella IgG; 92% vs. 92% for CMV IgG; and 98% vs. 98% for HSVI/II IgG. As shown in Table 1, the positive agreement of each antigen in Array-ELISA was high (>99.41%), while the negative agreements ranged from 87.5% to 99.75%. However, there was no significant statistical difference between rubella IgG and HSVI/II IgG.
The BGI-Array ELISA TORCH IgG assays were estimated to yield a TAT of about 1.5 hr for analysis of 100 samples for all four analyses. In contrast, testing by Virion/Serion ELISA by using a single processing instrument required about 5 hr for analysis and reporting of all four analyses. These TAT calculations translated into an approximate sample throughput of 600 samples by BGI-Array ELISA and 180 samples by Virion/Serion ELISA during a 9-hr shift. The procedure of BGI-Array ELISA is similar to the Virion/Serion ELISA method. Therefore, the four analyses of TORCH IgG by using routine ELISA assays will cost three times as much as BGI-Array ELISA. Moreover, these values do not account for instrumentation or associated personnel costs.
Prenatal TORCH screening is a routine practice in many parts of the world that greatly reduces the risk of transmitting viral or protozoan infections to the fetus in utero. Although TORCH infections are a significant cause of morbidity and mortality worldwide [3], the implementation of widespread TORCH screening has been hindered by the lack of consistent and reliable serologic methods [5]. In this study, we used a classical routine TORCH ELISA as a reference method. The prevalence of TORCH pathogens (BGI-Array ELISA vs. Virion/Serion ELISA) were 1.25% vs. 1.0% for toxoplasma IgG, 85% vs. 84.25% for rubella IgG, both 92% for CMV IgG and both 98% for HSVI/II IgG. Moreover, the data presented in this report indicate that the BGI-Array ELISA TORCH IgG assays show a near-perfect agreement (κ=0.8-0.93) to routine testing by Virion/Serion ELISA method. However, despite comparable overall performance, there were several differences in the test performance that should be discussed. Most importantly, the specificity of BGI-Array ELISA was lower than that of the Virion/Serion ELISA method, especially for HSV IgG (87.5%) and rubella IgG (91.94%), and these two antigens showed no statistical difference (P>0.05). This might be due to the high prevalence of HSV IgG (97.75%) and rubella IgG (83.75%), and the low negative numbers, which will generate a larger difference between these two methods. Moreover, it might also be due to the different antigens used in the BGI-Array ELISA and Virion/Serion ELISA kits.
BGI-Array ELISA showed good specificity and sensitivity with standard materials. Nine standard IgG antibodies to Toxoplasma gondii (P1-P9), 20 anti-rubella virus IgG antibodies (R1-R20), 17 anti-HSVI (HI1-HI17), and 9 HSVII (HII1-HII9) antibodies were all detected by the BGI-Array ELISA method. These results demonstrate the good antigen recognition ability of BGI-Array ELISA, which is sufficient for the clinical application of TORCH IgG screening.
A previous study has reported a protein microchip-based TORCH screening method [6]. However, the BGI-Array ELISA method integrates the ELISA method with protein microchip technology, making it more easily applied in clinical diagnostics. Moreover, the BGI-Array ELISA method has a higher throughput than the conventional ELISA method for TORCH screening test. The TAT for each sample showed great improvement between the BGI-Array ELISA and Virion/Serion ELISA methods (1.5 hr vs. 5 hr). On the other hand, the protocol of this novel BGI-Array ELISA method is similar to the routine ELISA method with more convenient manipulation characteristics and easy-to-use software. The BGI-Array ELISA method also shows convenient manipulation and similar high throughput and cost-efficiency to other high throughput methods, such as MFI. Therefore, this method is likely to be easily adopted in most clinical laboratories. However, this method also has some limitations. Firstly, it is a qualitative method for TORCH IgG screening, and the ODs do not show a good correlation with the titer of antibodies. Secondly, TORCH IgM screening also provides important information for clinical diagnosis but BGI-Array ELISA can only detect IgG antibodies. This is mainly due to the antigen and methodology design, and an Array of TORCH IgM is under development.
In conclusion, this BGI-Array ELISA TORCH IgG screening test is an easy-to-perform and high throughput method, showing a near-perfect agreement with conventional ELISA methods.
Acknowledgements
We thank Dr. Li Xin from BGI-GBI for the technical assistance. We also thank the grants of the Nature & Science projects in Suzhou City (SYSD2010136 & SYSD2009128).
References
1. TORCH syndrome and TORCH screening. Lancet. 1990; 335:1559–1561. PMID: 1972489.
2. Newton ER. Diagnosis of perinatal TORCH infections. Clin Obstet Gynecol. 1999; 42:59–70. quiz 174-5. PMID: 10073301.

3. Stegmann BJ, Carey JC. TORCH Infections. Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes infections. Curr Womens Health Rep. 2002; 2:253–258. PMID: 12150751.
4. Boutall A, Urban MF, Stewart C. Diagnosis, etiology, and outcome of fetal ascites in a South African hospital. Int J Gynaecol Obstet. 2011; 115:148–152. PMID: 21798534.

5. Binnicker MJ, Jespersen DJ, Harring JA. Multiplex detection of IgM and IgG class antibodies to Toxoplasma gondii, rubella virus, and cytomegalovirus using a novel multiplex flow immunoassay. Clin Vaccine Immunol. 2010; 17:1734–1738. PMID: 20861325.
6. Jiang L, Yu Z, Tang Z, Jiang T, Zhang C, Lu Z. Protein arrays based on biotin-streptavidin system for the simultaneous detection of TORCH infections. J Nanosci Nanotechnol. 2008; 8:2286–2292. PMID: 18572639.

7. Owen WE, Martins TB, Litwin CM, Roberts WL. Performance characteristics of six IMMULITE 2000 TORCH assays. Am J Clin Pathol. 2006; 126:900–905. PMID: 17074686.

8. Jiang L, Yu Z, Du W, Tang Z, Jiang T, Zhang C, et al. Development of a fluorescent and colorimetric detection methods-based protein microarray for serodiagnosis of TORCH infections. Biosens Bioelectron. 2008; 24:376–382. PMID: 18524564.

9. Mezzasoma L, Bacarese-Hamilton T, Di Cristina M, Rossi R, Bistoni F, Crisanti A. Antigen microarrays for serodiagnosis of infectious diseases. Clin Chem. 2002; 48:121–130. PMID: 11751547.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download