Abstract
There have been a number of studies about correlations between HLA genotypes in various ethnic groups and occurrence of various cutaneous adverse drug reactions, ranging in intensity from mild to severe, caused by antiepileptic drugs (AEDs). This is the first report analyzing the HLA genotypes of 9 Korean patients with skin rashes induced by various AEDs. The AEDs that induced skin rash were lamotrigine (n=3), carbamazepine (n=3), oxcarbazepine (n=1), phenobarbital (n=1), and phenytoin (n=1). None of the patients' HLA genotypes was either HLA-B*1502 or HLA-A*3101. Based on these series of cases, AED-induced skin rash can occur independently of HLA-B*1502 or HLA-A*3101 genotypes in the Korean patients.
Antiepileptic drugs (AEDs), especially drugs with aromatic structures, such as carbamazepine (CBZ), phenytoin (PHT), oxcarbazepine (OXC), phenobarbital (PB), and lamotrigine (LTG), are among the most common causes of cutaneous adverse drug reactions (cADRs) [1]. The cADRs caused by these aromatic AEDs range from mild maculopapular eruption (MPE) to potentially life-threatening reactions such as Stevens-Johnson syndrome or toxic epidermal necrolysis (SJS/TEN). The overall incidence of the array of cADRs caused by AEDs is approximately 10%, and the use of AEDs is often limited because of the frequency with which these reactions are encountered [1].
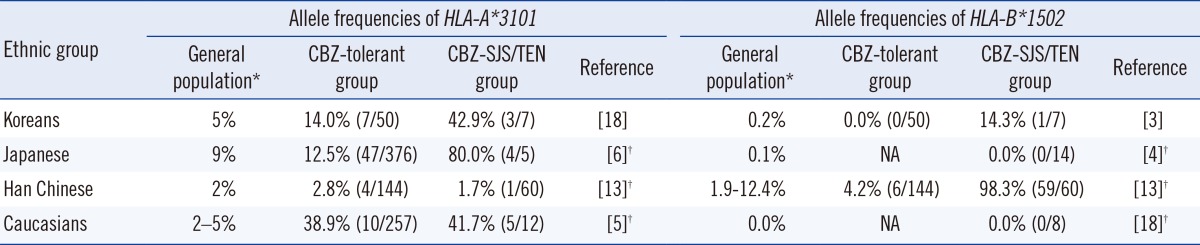
Strong associations between CBZ-induced SJS/TEN and the HLA-B*1502 allele have been identified in Han Chinese, Malay, Thai, and Indian populations, but not in Caucasian, Japanese, and Korean populations [2, 3, 4]. Instead, in these latter groups, the HLA-A*3101 allele was reported to be a genetic risk factor for all types of CBZ-induced cADRs in Caucasians [5] and Japanese [6], and for severe CBZ-induced cADRs in Koreans [3], with odds ratios of 9.12, 9.5, and 7.3, respectively.
Skin rash is not a life-threatening adverse drug reaction, but is of concern in AED therapy. Its occurrence is not uncommon, especially in patients who are prescribed aromatic AEDs (such as PHT, CBZ, and PB), with a frequency ranging from 5% to 15% in these treated patients [7]; it often requires discontinuation of the causative drug, or results in patient non-compliance with the treatment regimen, leading to loss of control over seizure activity [8]. Several studies on HLA genotypes in Chinese patients with AED-induced skin rash have suggested an association of the HLA-B*1502 allele with OXC-induced skin rash [9], but not with CBZ-induced or LTG-induced skin rash [2, 10]. These findings imply that the association between HLA-B*1502 and AED-induced cADR is specific to CBZ, to Southeast Asian patients, and to SJS/TEN.
To date, there has been only one study about the HLA genotypes of Korean patients with CBZ-induced severe cADR; the association of HLA genotypes with mild cADR, such as skin rash, has not been investigated in Korean patients. In addition, the association of HLA genotypes with reactions to assorted AEDs beyond CBZ has not been examined, even though the use of the newer AEDs, including OXC and LTG, is increasing. Therefore, we report 9 cases of Korean patients with skin rashes induced by a variety of AEDs, along with their HLA genotypes.
We retrospectively enrolled patients of Korean ethnicity who have experienced AED-induced skin rash in our institution from 2007 to 2012, and total 9 patients were recruited for this study. AED-induced skin rash was defined according to the following criteria: 1) occurrence within 12 weeks after first exposure to one of the implicated drugs [11]; 2) manifestation of a cutaneous nature, without tenderness, and with a spotty, non-confluent, morbilliform, or maculopapular appearance [8]; 3) exclusion of all other etiologies causing the cutaneous manifestations; and 4) amelioration of all cutaneous manifestations concurrent with withdrawal of the implicated drug. Peripheral blood was collected from all study subjects, written informed consent was obtained, and genomic DNA was extracted from peripheral blood mononuclear cells. We determined the HLA-A and HLA-B genotypes by PCR using a sequence-specific primer kit (Abbot Laboratories; Abbot Park, IL, USA). The serological equivalents of HLA-A*31 and HLA-B*15 alleles were assigned according to the most recently published literature about HLA alleles identified in Koreans [12]. Our study was conducted with the approval of the Institutional Review Board of the Samsung Medical Center (IRB 2014-03-081-002).
The age ranged from 16 to 74 yr, and the AEDs implicated in causing the rashes were LTG (n=3), CBZ (n=3), OXC (n=1), PB (n=1), and PHT (n=1). The prescribed doses of AEDs were not significantly above their generally recommended ranges for any patients; similarly, the serum drug concentrations were not significantly above their therapeutic ranges in any patients. Latency period to cutaneous manifestations after first exposure to the AEDs ranged from 1 day to a maximum of 51 days. Regarding HLA-A genotype, none of the 9 subjects carried the HLA-A*31 allele. Regarding HLA-B genotype, 2 of the 9 subjects carried the HLA-B*15 allele. Serologic equivalents of HLA-B*15 alleles from both patients were of the B62 serotype [12], which is associated with HLA-B*1501, B*1507, and B*1527 alleles, not with the HLA-B*1502 allele. None of the 9 subjects carried the HLA-A*3101 allele or the HLA-B*1502 allele. Clinical characteristics and HLA genotypes of the 9 patients are summarized in Table 1.
All 9 patients discontinued the AED that caused the rash and switched over to other drugs. One patient, who experienced CBZ-induced skin rash, also exhibited periorbital numbness when treated with valproate (VPA). Another patient experienced hypersensitivity-related drug reactions such as skin symptoms and neuropathy, to most AEDs (including PB, CBZ, LTG, pregabalin, topiramate, diazepam, and clobazam). The rest of the patients did not display any hypersensitivity-related drug reactions after switching to non-aromatic AEDs such as VPA, topiramate, or clobazam.
It is likely that the specific cutaneous phenotype, as well as ethnicity, is relevant to the degree of correlation between AED-induced cADR and HLA genotype. A study of Han Chinese by Hung et al. [13] showed that CBZ-induced MPE was not associated with the HLA-B*1502 allele, while the HLA-A*3101 allele was related, with an odds ratio of 17.5, to CBZ-induced MPE. A previous study of Korean patients by Kim et al. [3] showed that the HLA-B*1511 and HLA-A*3101 alleles were associated with CBZ-induced SJS and hypersensitivity syndrome/severe cADR, respectively, but not with HLA-B*1502 allele. Likewise, in this study, none of 3 subjects with CBZ-induced skin rash carried the HLA-B*1502 allele.
Cross reactivity among aromatic amine AEDs such as CBZ, PHT, PB, OXC, and LTG is observed with a high frequency in AED-induced cADR patients, and symptoms of hypersensitivity were reported twice as frequently with aromatic AEDs as with non-aromatic AEDs [7, 14]. In a case-control association study in Taiwanese patients with SJS/TEN, the odds ratios of the correlation between the HLA-B*1502 allele and a reaction to PHT, LTG, and OXC were 5.1, 5.1, and 80.7, respectively, indicating that individuals with this allele share an increased risk for adverse reactions to any one of multiple aromatic anticonvulsants [15]. In cases of skin rash, rates of cross-reactivity between certain AEDs, especially when involving CBZ and PHT, were also high [7]. With the exception of 2 patients, subjects in this study showed no cross-reactivity between aromatic AEDs and non-aromatic AEDs, and cross-reactivity between aromatic AEDs could not be determined. We were unable to perform a case-control study to identify alleles that confer an increased predisposition to aromatic AED-induced skin rash in Koreans, or to evaluate odds ratios for any particular HLA allele; such a case-control study could yield valuable results.
The genetic susceptibility to AED-induced cADR appears to be ethnicity-specific, and associations between HLA alleles and cADR are dependent on the HLA allele frequencies in the general populations (Table 2). For example, the prevalence of the HLA-A*3101 allele is relatively high in the Japanese population, where the HLA-A*3101 allele is likely to be a marker for CBZ-induced SJS/TEN. Similarly, the prevalence of the HLA-B*1502 allele is extremely high in the Han Chinese population, where the HLA-B*1502 allele is, most certainly, a marker for CBZ-induced SJS/TEN. In the Korean population, the HLA-A alleles A*0201, A*1101, A*2402, and A*3303, and the HLA-B alleles B*1501, B*3501, B*4403, B*5101, B*5401, and B*5801, showed the highest frequencies [16], and further evaluation of these alleles might be helpful in the search for genetic associations.
In conclusion, this is the first report on the HLA genotypes in the Korean patients with skin rashes induced by various AEDs. We found neither HLA-A*3101 nor HLA-B*1502 alleles in our cases. This series of cases suggests that AED-induced skin rash can occur independently of HLA-B*1502 or HLA-A*3101 genotypes in Korean patients. Further case-control studies on patients with AED-induced skin rash should be performed to determine whether any association exists between HLA genotype and AED-induced skin rash, as well as to search for other genetic associations.
Acknowledgments
This work was supported by the Samsung Biomedical Research Institute grant (#SBRI GL1B22111).
References
1. Chung WH, Hung SI, Chen YT. Genetic predisposition of life-threatening antiepileptic-induced skin reactions. Expert Opin Drug Saf. 2010; 9:15–21. PMID: 20001755.

2. Chong KW, Chan DW, Cheung YB, Ching LK, Hie SL, Thomas T, et al. Association of carbamazepine-induced severe cutaneous drug reactions and HLA-B*1502 allele status, and dose and treatment duration in paediatric neurology patients in Singapore. Arch Dis Child. 2014; 99:581–584. PMID: 24225276.
3. Kim SH, Lee KW, Song WJ, Kim SH, Jee YK, Lee SM, et al. Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. 2011; 97:190–197. PMID: 21917426.

4. Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K, et al. HLA-B*1511 is a risk factor for carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Epilepsia. 2010; 51:2461–2465. PMID: 21204807.

5. McCormack M, Alfirevic A, Bourgeois S, Farrell JJ, Kasperavičiūtė D, Carrington M, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011; 364:1134–1143.

6. Ozeki T, Mushiroda T, Yowang A, Takahashi A, Kubo M, Shirakata Y, et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet. 2011; 20:1034–1041. PMID: 21149285.

7. Wang XQ, Lang SY, Shi XB, Tian HJ, Wang RF, Yang F. Cross-reactivity of skin rashes with current antiepileptic drugs in Chinese population. Seizure. 2010; 19:562–566.

8. Zaccara G, Franciotta D, Perucca E. Idiosyncratic adverse reactions to antiepileptic drugs. Epilepsia. 2007; 48:1223–1244.

9. Hu FY, Wu XT, An DM, Yan B, Stefan H, Zhou D. Pilot association study of oxcarbazepine-induced mild cutaneous adverse reactions with HLA-B*1502 allele in Chinese Han population. Seizure. 2011; 20:160–162. PMID: 21169036.

10. Shi YW, Min FL, Liu XR, Zan LX, Gao MM, Yu MJ, et al. Hla-B alleles and lamotrigine-induced cutaneous adverse drug reactions in the Han Chinese population. Basic Clin Pharmacol Toxicol. 2011; 109:42–46.

11. Gogtay NJ, Bavdekar SB, Kshirsagar NA. Anticonvulsant hypersensitivity syndrome: a review. Expert Opin Drug Saf. 2005; 4:571–581.

12. Lee KW, Park MH. [New HLA nomenclature (2010) and its clinical application in Koreans]. Korean J Lab Med. 2010; 30:203–217.

13. Hung SI, Chung WH, Jee SH, Chen WC, Chang YT, Lee WR, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics. 2006; 16:297–306.

15. Hung SI, Chung WH, Liu ZS, Chen CH, Hsih MS, Hui RC, et al. Common risk allele in aromatic antiepileptic-drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics. 2010; 11:349–356. PMID: 20235791.

16. Lee KW, Oh DH, Lee C, Yang SY. Allelic and haplotypic diversity of HLA-A, -B, -C, -DRB1, and -DQB1 genes in the Korean population. Tissue Antigens. 2005; 65:437–447.

17. Burtis C, Ashwood E, Bruns D, editors. Tietz Textbook of clinical chemistry and molecular diagnostics. 5th ed. St. Louis, MO: Elsevier;2012.
18. Lonjou C, Thomas L, Borot N, Ledger N, de Toma C, LeLouet H, et al. A marker for Stevens-Johnson syndrome...: ethnicity matters. Pharmacogenomics J. 2006; 6:265–268.
Table 1
Summary of clinical characteristics and HLA genotypes in the 9 study subjects
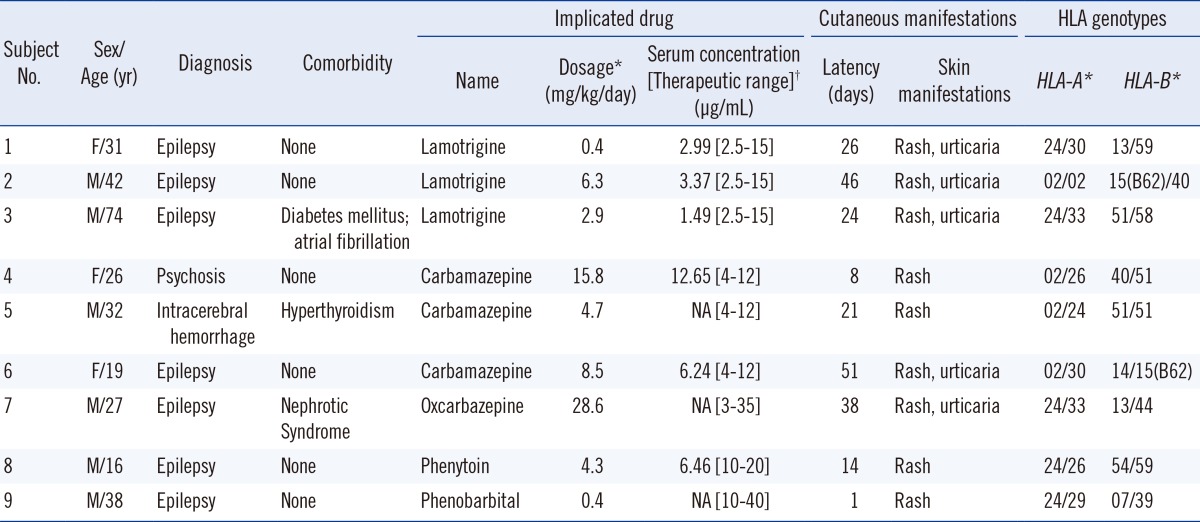
*At the occurrence of cutaneous manifestation; †From Tietz, textbook of clinical chemistry, 5th edition [17].
Abbreviations: F, female; M, male; NA, data not available; 15(B62), allele HLA-B15, serotype B62.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download