Abstract
Background
Neutrophil gelatinase-associated lipocalin (NGAL) is a promising biomarker in the detection of kidney injury. Early diagnosis of urinary tract infection (UTI), one of the most common infections in children, is important in order to avert long-term consequences. We assessed whether serum NGAL (sNGAL) or urine NGAL (uNGAL) would be reliable markers of UTI and evaluated the appropriate diagnostic cutoff value for the screening of UTI in children.
Methods
A total of 812 urine specimens and 323 serum samples, collected from pediatric patients, were analyzed. UTI was diagnosed on the basis of culture results and symptoms reported by the patients. NGAL values were measured by using ELISA.
Results
NGAL values were more elevated in the UTI cases than in the non-UTI cases, but the difference between the values were not statistically significant (P=0.190 for sNGAL and P=0.064 for uNGAL). The optimal diagnostic cutoff values of sNGAL and uNGAL for UTI screening were 65.25 ng/mL and 5.75 ng/mL, respectively.
Neutrophil gelatinase-associated lipocalin (NGAL) is a 25 kDa protein and a member of the lipocalin family [1]. NGAL is covalently bound to matrix metalloproteinase 9 expressed by neutrophils and exhibits antibacterial properties, and so, this lipocalin is considered a component of the innate immune system [2]. NGAL is also expressed by other cells, such as kidney epithelial and tubular cells [1, 3]. Although NGAL is expressed only at very low values in several human tissues, its expression is markedly increased in injured epithelial cells, including in those of the kidney, colon, liver, and lung [1]. These findings provide a potential molecular mechanism for the role of NGAL in affecting the epithelial phenotype, both during kidney development and following acute kidney injury (AKI) [4].
Urinary tract infection (UTI) is one of the most common infections in children. Early diagnosis of UTI is important because delayed diagnosis may result in the failure of subsequent treatment and may result in long-term consequences, including renal scarring, hypertension, and chronic renal failure [5]. Urine culture is the standard approach for the diagnosis of UTI, but it has limitations. Difficulty in specimen collection and interpretation of inadequately collected specimens may contribute to misdiagnosis [5]. Furthermore, positive culture results require 2-3 days in order to identify the cause of the infection. Despite these limitations, urinalysis is commonly used in screening for UTI. However, indicators of UTI in urinalysis, such as pyuria and positive nitrite test results, have some limitations associated with low sensitivity [5]. Nitrite tests produce false negative results in the presence of gram-positive pathogens, and the sensitivity of this test can be decreased when testing urine with a high specific gravity. Similarly, pyuria may be absent in Proteus species infections. In addition, sterile pyuria may occur in noninfectious conditions such as urolithiasis [5]. Therefore, another marker is needed for rapid and accurate diagnosis of UTI to initiate early treatment.
Several studies have suggested that urine NGAL (uNGAL) may be associated with UTI [6, 7, 8]. In addition, since UTI can cause neutrophilia, we attempted to evaluate the association between serum NGAL (sNGAL) and UTI. The aim of this study was to assess whether sNGAL and/or uNGAL could be used as reliable UTI markers and to evaluate the appropriate NGAL detection cutoff values for screening of UTI in children.
This retrospective study was conducted among children who visited Chung-Ang University hospital in Seoul, Korea between February and November 2010. Serum and/or catheterization or midstream urine samples were obtained. Urine culture, urinalysis, serum blood urea nitrogen (BUN) and creatinine (Cr), C-reactive protein (CRP), and whole blood white blood cell (WBC) count results were retrospectively reviewed.
A total of 812 urine specimens from 333 patients and 323 serum samples from 208 patients were collected from a total of 444 patients (177 males, 267 females). Both serum and urine specimens were available from 97 patients. Either serum or urine samples were available from the remaining 347 patients. The UTI group consisted of 107 children (58 males, 49 females), and 191 serum samples and 284 urine samples were included. The mean age of the UTI group was 4.29±2.93 yr. The non-UTI group consisted of 337 children (119 males, 218 females), and 232 serum samples and 528 urine samples were included. The mean age of the non-UTI group was 3.87±2.59 yr. Serum BUN and Cr levels were normal in all children in both groups. Whole blood WBC count did not show a significant difference in NGAL values between the UTI group and the non-UTI group (12.4×109/L vs. 10.7×109/L, P=0.087). Serum CRP level was significantly higher in the UTI group than in the non-UTI group (3.20 mg/L vs. 0.79 mg/L, P=0.042).
Urinalysis was performed using URiSCAN (YD Diagnostics, Seoul, Korea), and microscopic analysis of urine was performed by using a Sysmex UF-1000i full automatic urine analyzer (Sysmex, Hyogo, Japan). Values of sNGAL and uNGAL were assayed by using a NGAL Rapid ELISA Kit (Bioporto diagnostics, Gentofte, Denmark) following the manufacturer's instructions. Bioporto's NGAL Rapid ELISA Kit assay is a sandwich ELISA performed in microwells coated with an anti-human NGAL monoclonal antibody. Bound NGAL is detected with another monoclonal antibody labeled with biotin, and the assay is developed with horseradish peroxidase (HRP)-conjugated streptavidin and a color-forming substrate. The color intensity is read at 450 nm using PR 3100 TSC Microplate Reader (Bio-Rad Laboratories, Hercules, CA, USA).
UTI was diagnosed by significant bacteriuria (≥100,000 colony-forming unit [CFU]/mL of a single pathogen) without symptoms associated with UTI, or by significant bacteriuria (≥10,000 CFU/mL) with symptoms associated with UTI, including any of the followings: fever, vomiting, dysuria, abdominal pain, nausea, and voiding frequency [9]. Additionally, we defined our own criteria for the screening of presumptive UTI (pUTI) by urinalysis markers. First, if pyuria (WBC ≥5/HPF) were present, a diagnosis of pUTI was made. Second, if leukocyte esterase was present, patients were regarded as having pUTI.
Statistical analysis was performed by using the IBM SPSS Statistics for Windows, version 19 (SPSS Inc., Chicago, IL, USA). The independent samples t-test was used to compare mean NGAL values in both groups. ROC analysis was performed to determine the sensitivity, specificity, and optimal diagnostic cutoff values of NGAL in screening for UTI. The most appropriate cutoff value was chosen according to ROC analysis, and the area under the curve (AUC) was calculated. The statistical significance level was established at P<0.05.
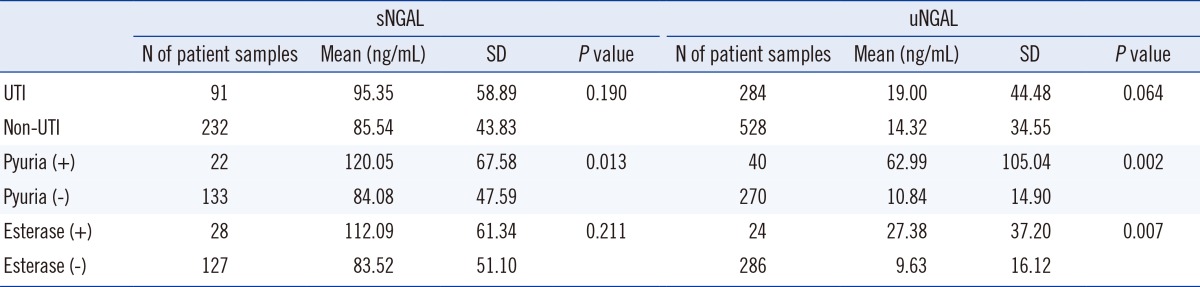
The values of sNGAL and uNGAL were elevated in the UTI group compared to the non-UTI group; however, there was no significant difference in the mean values between the groups (Table 1). According to ROC analysis, the optimal diagnostic cutoff values to predict UTI were 65.25 ng/mL for sNGAL and 5.75 ng/mL for uNGAL. If a cutoff of 65.25 ng/mL for sNGAL for diagnosing UTI was used, the sensitivity and specificity of the diagnosis were 70% (95% confidence interval [CI], 18%-79%) and 35% (95% CI, 20%-74%), respectively (Fig. 1). For uNGAL, using a cutoff value of 5.75 ng/mL for diagnosing UTI, the sensitivity and specificity of diagnosis were 70% (95% CI, 21%-83%) and 42% (95% CI, 36%-89%), respectively (Fig. 1).
The results of the pUTI and the non-pUTI groups based on the presence or absence of pyuria are presented below. Mean values of sNGAL were significantly higher in the pyuria-positive pUTI group than in the pyuria-negative group (120.05 ng/mL vs. 84.08 ng/mL, P=0.013) (Table 1). Mean uNGAL values were also significantly higher in the pyuria-positive pUTI group than in the pyuria-negative, non-pUTI group (67.58 ng/mL vs. 47.59 ng/mL, P=0.007) (Table 1). According to ROC analysis, the optimal diagnostic cutoff value was 82.5 ng/mL for sNGAL, with 70% sensitivity (95% CI, 23%-86%) and 60% specificity (95% CI, 27%-88%) (Fig. 2). Similarly, the optimal diagnostic cutoff value was 10.3 ng/mL for uNGAL, with 70% sensitivity (95% CI, 20%-87%) and 70% specificity (95% CI, 21%-86%) (Fig. 2).
In examining the presence or absence of leukocyte esterase in the pUTI and non-pUTI group samples, mean sNGAL and uNGAL values were found to be higher in the esterase-positive pUTI group than the esterase-negative, non-pUTI group (112.09 ng/mL vs. 83.52 ng/mL, 27.38 ng/mL vs. 9.63 ng/mL), but only the mean uNGAL value between the 2 groups showed a statistically significant difference (P=0.007) (Table 1). According to ROC analysis, the optimal diagnostic cutoff value to predict pUTI was 75.05 ng/mL for sNGAL and 9.65 ng/mL for uNGAL. Using a cutoff of 75.05 ng/mL of sNGAL for diagnosing pUTI, the sensitivity and specificity were 70% (95% CI, 24%-89%) and 49% (95% CI, 11%-62%), respectively (Fig. 3). For uNGAL, using a cutoff value of 9.65 ng/mL for the presumptive diagnosis of UTI, the sensitivity and specificity were 70% (95% CI, 30%-91%) and 68% (95% CI, 25%-78%), respectively (Fig. 3).
Lastly, we evaluated the uNGAL:Cr ratio between the culture-proven UTI and non-UTI groups. There was no significant difference in uNGAL:Cr ratio between the 2 groups (results not shown).
NGAL is a useful marker for the early diagnosis of AKI [1, 4, 10, 11, 12, 13, 14] and several studies have evaluated uNGAL values in the early diagnosis of UTI [3, 6, 7, 8, 15]. Ichino et al. [3] reported increased uNGAL value in pyelonephritis using an experimental rat model, and Yilmaz et al. [4] reported that uNGAL and uNGAL:Cr ratio were useful markers in the early diagnosis of UTI in children without acute renal injury and/or chronic kidney disease. In contrast, we did not find significant differences between sNGAL and uNGAL values in the presence or absence of UTI. NGAL was originally isolated from human neutrophils; however, subsequent studies demonstrated that NGAL may also be expressed under certain conditions in cells in the kidney and liver, and in epithelial tissues [16, 17]. Increased values of sNGAL and uNGAL are observed in AKI [1]. With respect to sNGAL, AKI results in increased NGAL mRNA expression in distal organs [18], specifically in the liver and lungs, and over-expressed NGAL protein released into the circulation may contribute to increases in sNGAL values [1]. In addition, NGAL is an acute phase reactant and may be released from neutrophils, macrophages, and other immune cells [1]. Furthermore, decreases in glomerula filtration rate resulting from AKI would be expected to decrease the renal clearance of NGAL, resulting in its subsequent accumulation in the serum [1]. sNGAL is freely filtered by the glomerulus and is largely reabsorbed in the proximal tubules [19], whereas uNGAL is detected only in the presence of a concomitant proximal renal tubular injury that precludes NGAL reabsorption and/or increases NGAL synthesis [1]. Recent studies revealed that uNGAL is increased in AKI, and it has been speculated that NGAL expression is induced in order to aid in tissue regeneration after kidney damage [3, 11]. The contribution of neutrophilic NGAL to uNGAL values is generally small, but the effect may be important in pyuria [8]. Theoretically, sNGAL or uNGAL values may not increase during UTI in the absence of upper urinary tract injury; even in the presence of UTI, pyuria is not always observed. Therefore, it is possible that there is no association between UTI and increased NGAL values.
Since sNGAL or uNGAL values alone did not sufficiently predict culture-proven UTI, we compared NGAL values in the presence or absence of pyuria or urine leukocyte esterase. Decavele et al. [8] demonstrated that urine WBC counts are significantly correlated with uNGAL values in both upper and lower UTI. In agreement with this finding, in the presence of pyuria or urine leukocyte esterase, the uNGAL value was significantly higher than in their absence. These results can be explained by the presence of NGAL originating from urinary neutrophils [8]. sNGAL value was significantly higher only in the presence of pyuria. We speculate that this was due to the difference in diagnostic cutoff sensitivity in the measurements taken in the presence of pyuria and leukocyte esterase. Such results suggest that uNGAL value is more useful than sNGAL during screening for UTI.
For more rapid diagnosis of UTI, many clinicians routinely use urinalysis results, such as the presence of pyuria, nitrite, and leukocyte esterase, but the low sensitivity of this analysis is well known. One study showed that sensitivity and specificity of leukocyte esterase were 65.4% and 94%, respectively, whereas sensitivity and specificity of the nitrite test were 38.9% and 99.5%, respectively [15]. In this study, we demonstrated the optimal cutoff values for sNGAL (65.25 ng/mL) and uNGAL (5.75 ng/mL) in the diagnosis of UTI. At these established cutoff points, the sensitivity in the measurement of both sNGAL and uNGAL (70%) was found to be higher than in the measurement of leukocyte esterase or in the nitrite test, although the specificity of sNGAL (35%) and uNGAL (42%) was lower than in urinalysis. Hirsch et al. [11] reported that AKI due to contrast administration can be predicted using a cutoff of 100 ng/mL for uNGAL. In different studies, the cutoff values for predicting AKI after cardiopulmonary bypass were determined to be between 50 ng/mL and 100 ng/mL [10, 12]. Moreover, according to the manufacturer, the cutoff value for sNGAL was 106 ng/mL and 9.8 ng/mL for uNGAL. Our results suggest that the optimal cutoff value for predicting UTI is lower than the values determined for AKI and even lower than the values suggested by the manufacturer.
In screening for pUTI using pyuria or urine leukocyte esterase values, we evaluated the optimal concentration of sNGAL and uNGAL for such cases. sNGAL values were consistently lower than the manufacturer's reports (Bioporto diagnostics; 82.5 ng/mL and 75.05 ng/mL) at a sensitivity of 70%. uNGAL values were also similar to or lower than the manufacturer's reports (Bioporto; 10.3 ng/mL and 9.65 ng/mL). At these values, specificity was higher than that of culture-proven UTI (70% in the pyuria-positive group and 68% in the esterase-positive group). Therefore, we confirmed that uNGAL value is correlated with the presence of leukocytes in the urine sample.
In conclusion, to the best of our knowledge, this is the first study demonstrating that there is no significant correlation between sNGAL or uNGAL values and UTI. We suggest that the use of NGAL as a new marker in the early diagnosis of UTI may not be appropriate. If NGAL is being considered as a marker in the early diagnosis of UTI, uNGAL value will be a more useful marker than sNGAL value. In addition, a new cutoff value must be set that is lower than the value reported by the manufacturer in order to increase sensitivity. To better understand the relationship between NGAL values and UTI in children, and to set an appropriate diagnostic cutoff value of NGAL in the early diagnosis of UTI in children, a future prospective study using larger numbers of patients and a control group of healthy children is needed.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0014896).
References
1. Devarajan P. Review: neutrophil gelatinase-associated lipocalin: a troponin-like biomarker for human acute kidney injury. Nephrology. 2010; 15:419–428. PMID: 20609093.

2. Fjaertoft G, Foucard T, Xu S, Venqe P. Human neutrophil lipocalin (HNL) as a diagnostic tool in children with acute infections: a study of the kinetics. Acta Paediatr. 2005; 94:661–666.

3. Ichino M, Kuroyanagi Y, Kusaka M, Mori T, Ishikawa K, Shiroki R, et al. Increased urinary neutrophil gelatinase associated lipocalin levels in a rat model of upper urinary tract infection. J Urol. 2009; 181:2326–2331. PMID: 19303090.

4. Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, et al. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004; 15:3073–3082.

5. Jantausch B, Kher K. Urinary tract infection. In : Kher KK, Schnaper HW, Makker SP, editors. Clinical paediatric nephrology. 2nd ed. UK: Informa UK;2007. p. 553–573.
6. Yilmaz A, Sevketoglu E, Gedikbasi A, Karyagar S, Kiyak A, Mulazimoglu M, et al. Early prediction of urinary tract infection with urinary neutrophil gelatinase associated lipocalin. Pediatr Nephrol. 2009; 24:2387–2392. PMID: 19649660.

7. Hatipoglu S, Sevketoglu E, Gedikbasi A, Yilmaz A, Kiyak A, Mulazimoglu M, et al. Urinary MMP-9/NGAL complex in children with acute cystitis. Pediatr Nephrol. 2011; 26:1263–1268. PMID: 21556719.

8. Decavele AS, Dhondt L, De Buyzere ML, Delanghe JR. Increased urinary neutrophil gelatinase associated lipocalin in urinary tract infections and leukocyturia. Clin Chem Lab Med. 2011; 49:999–1003.

9. Behrman RE, Kliegman RM, Jenson HB, editors. Nelson Textbook of Pediatrics. 17th ed. Philadelphia: WB Saunders;2003. p. 1785–1790.
10. Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008; 3:665–673.

11. Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH 3rd, Ma Q, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007; 22:2089–2095.

12. Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005; 365:1231–1238.

13. Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007; 71:967–970.

14. Nguyen MT, Devarajan P. Biomarkers for the early detection of acute kidney injury. Pediatr Nephrol. 2008; 23:2151–2157. PMID: 17394022.

15. dos Santos JC, Weber LP, Perez LR. Evaluation of urinalysis parameters to predict urinary-tract infection. Braz J Infect Dis. 2007; 11:479–481.

16. Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997; 45:17–23. PMID: 9339356.

17. Friedl A, Stoesz SP, Buckley P, Gould MN. Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression. Histochem J. 1999; 31:433–441.
18. Grigoryev D, Liu M, Hassoun HT, Cheadle C, Barnes KC, Rabb H. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol. 2008; 19:547–558.

19. Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007; 18:407–413. PMID: 17229907.

Fig. 1
ROC curves to detect urinary tract infection (UTI). The blue line is ROC curve for serum neutrophil gelatinase-associated lipocalin (sNGAL) (area under the curve [AUC]=0.534; 95% CI, 0.308-0.812). By using a cutoff value of 65.25 ng/mL for the diagnosis of UTI, sensitivity and specificity were 70% and 35%, respectively (blue dot). The red line is ROC curve for urine neutrophil gelatinase-associated lipocalin (uNGAL) (AUC=0.576; 95% CI, 0.347-0.799). By using a cutoff value of 5.75 ng/mL for the diagnosis of UTI, sensitivity and specificity were 70% and 42%, respectively (red dot).
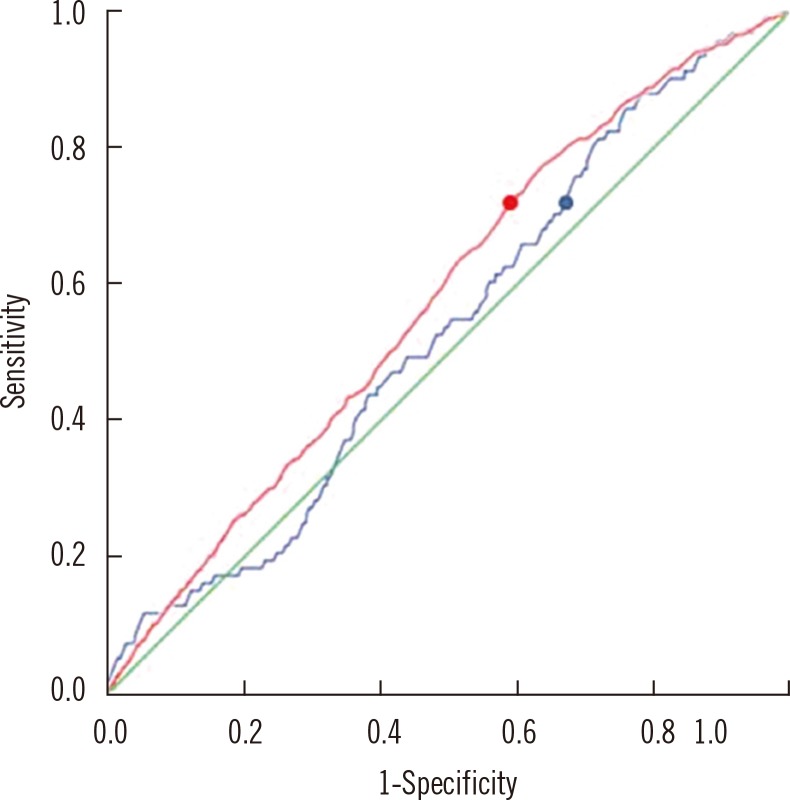
Fig. 2
ROC curves to detect urinary tract infection (UTI) defined by the presence or absence of pyuria. The blue line is ROC curve for serum neutrophil gelatinase-associated lipocalin (sNGAL) (area under the curve [AUC]=0.675; 95% CI, 0.372-0.845). By using a cutoff value of 82.5 ng/mL for the diagnosis of UTI, sensitivity and specificity were 70% and 60%, respectively (blue dot). The red line is ROC curve for urine neutrophil gelatinase-associated lipocalin (uNGAL) (AUC=0.780; 95% CI, 0.423-0.936). By using a cutoff value of 10.3 ng/mL for the diagnosis of UTI, sensitivity and specificity were 70% and 70%, respectively (red dot).
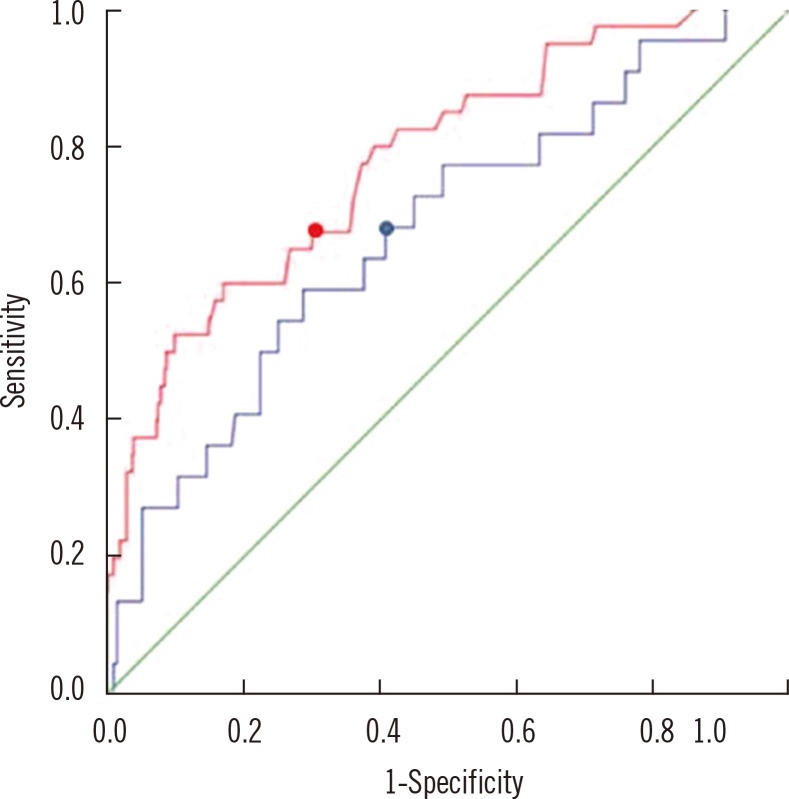
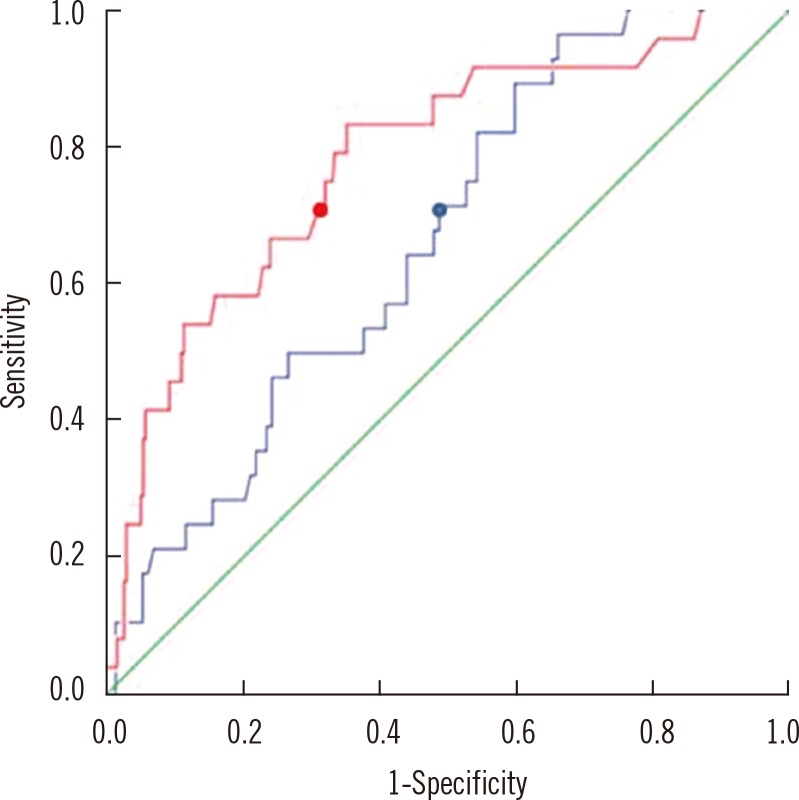
Fig. 3
ROC curves to detect urinary tract infection (UTI) defined by the presence or absence of leukocyte esterase. The blue line is ROC curve for serum neutrophil gelatinase-associated lipocalin (sNGAL) (area under the curve [AUC]=0.654; 95% CI, 0.397-0.922). By using a cutoff value of 75.05 ng/mL for the diagnosis of UTI, sensitivity and specificity were 70% and 49%, respectively (blue dot). The red line is ROC curve for urine neutrophil gelatinase-associated lipocalin (uNGAL) (AUC=0.761; 95% CI, 0.435-0.977). By using a cutoff value of 9.65 ng/mL for the diagnosis of UTI, sensitivity and specificity were 70% and 68%, respectively (red dot).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download