Abstract
Direct plating of synovial fluid (SF) on agar-based media often fails to identify pathogens in septic arthritis (SA). We developed a PCR assay for the simultaneous detection of Kingella kingae and Staphylococcus aureus from SF to evaluate molecular detection in SF and to estimate the incidence of K. kingae in SA in North America. The assay was based on detection of the cpn60 gene of K. kingae and the spa gene of S. aureus in multiplex real-time PCR. K. kingae was identified in 50% of patients between 0 and 5 yr of age (n=6) but not in any patients >18 yr old (n=105). Direct plating of SF on agar-based media failed to detect K. kingae in all samples. The PCR assay was inferior to the culture-based method for S. aureus, detecting only 50% of culture-positive cases. Our findings suggest that K. kingae is a common pathogen in pediatric SA in North America, in agreement with previous reports from Europe. PCR-based assays for the detection of K. kingae may be considered in children with SA, especially in those with a high degree of clinical suspicion.
Bacterial cultures of synovial fluid (SF) are often negative in suspected cases of septic arthritis (SA) [1, 2, 3]. While Staphylococcus aureus is the most common causative agent of culture-confirmed SA, Kingella kingae is increasingly being recognized as an important cause of SA in young children [3, 4]. S. aureus is readily isolated by using conventional culture-based techniques, although the inhibitory nature of SF or treatment with antibiotics prior to specimen collection may lead to false-negative results [5, 8]. K. kingae is a fastidious gram-negative bacterium that is rarely recovered by direct plating of SF on agar-based media, and recent PCR-based studies from Europe on SF specimens suggest that the rate of K. kingae SA in pediatric patients is grossly underestimated [4, 6, 7, 8, 9]. However, the incidence of K. kingae SA in North America remains unclear.
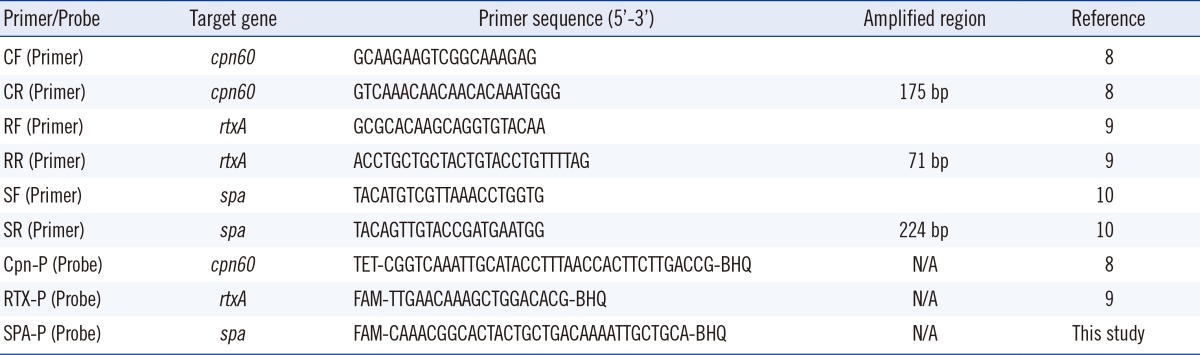
We developed a multiplex real-time PCR assay targeting the spa gene of S. aureus and the cpn60 and rtxA genes of K. kingae [8, 10]. Primers and probes were based on published literature and in-house bioinformatics analyses (Table 1). DNA was extracted from SF by using the MoBio BiOstic bacteremia DNA isolation kit (MO BIO Laboratories Inc., Carlsbad, CA, USA). The reaction volume for PCR was 25 µL, comprising 5 µL of DNA template, 12.5 µL of 2× Qiagen Quantifast PCR master mix (Qiagen, Valencia, CA, USA), 0.2 µmol of primers, and 0.2 µmol of probes. PCR reactions were run for 45 cycles of melting at 95℃ for 10 sec, annealing at 60℃ for 10 sec, and extension at 70℃ for 15 sec, by using the SmartCyclerII system (Cepheid, Sunnyvale, CA, USA). Fluorescence detection was performed at the annealing step.
Specificity of the assay was established by using DNA from bacteria showing genetic similarity to either K. kingae or S. aureus, and by using known agents of SA, including Moraxella catarrhalis, Haemophilus influenzae, Aggregatibacter actinomycetemcomitans, Micrococcus spp., Streptococcus pneumoniae, Staphylococcus epidermidis, Staphylococcus lugdunensis, Neisseria gonorrhoeae, Neisseria flavescens, Eikenella corrodens, S.aureus, and K. kingae. A threshold cycle (Ct) of 34 cycles was selected for reporting a "positive" detection value for K. kingae (for both cpn60 and rtxA) and 38 cycles for S. aureus detection. By using serial dilutions of DNA, the limit of detection was 56 fg of K. kingae DNA for both cpn60 and rtxA and 74.2 fg of S. aureus DNA for spa. Control culture-negative SF was spiked with serial dilutions of pure K. kingae (clinical isolate) or S. aureus (ATCC 29213). For K. kingae, the limit of detection was 1.5×103 colony-forming units (CFU)/mL for cpn60 and 1.5×104 CFU/mL for rtxA. For S. aureus, the limit of detection was 1.5×104 CFU/mL for spa. Given the superior detection limit for cpn60 over rtxA, cpn60, and spa were selected for simultaneous multiplex PCR detection of K. kingae and S. aureus.
SF samples were collected from 117 patients with suspected SA between 7/16/2009 and 5/29/2011 at 2 major academic medical centers in the United States: St. Louis Children's Hospital/Washington University School of Medicine (11 children and 1 adult) and the Vanderbilt University Hospital (1 child and 104 adults). Samples from the Vanderbilt University hospital were frozen and shipped to St. Louis, where all PCR testing was performed. All samples underwent standard analysis for SF, including direct plating on solid agar-based media at the source institution prior to PCR analysis.
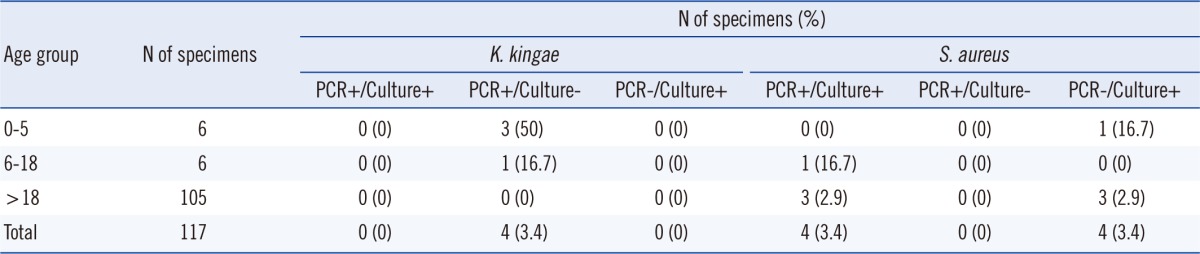
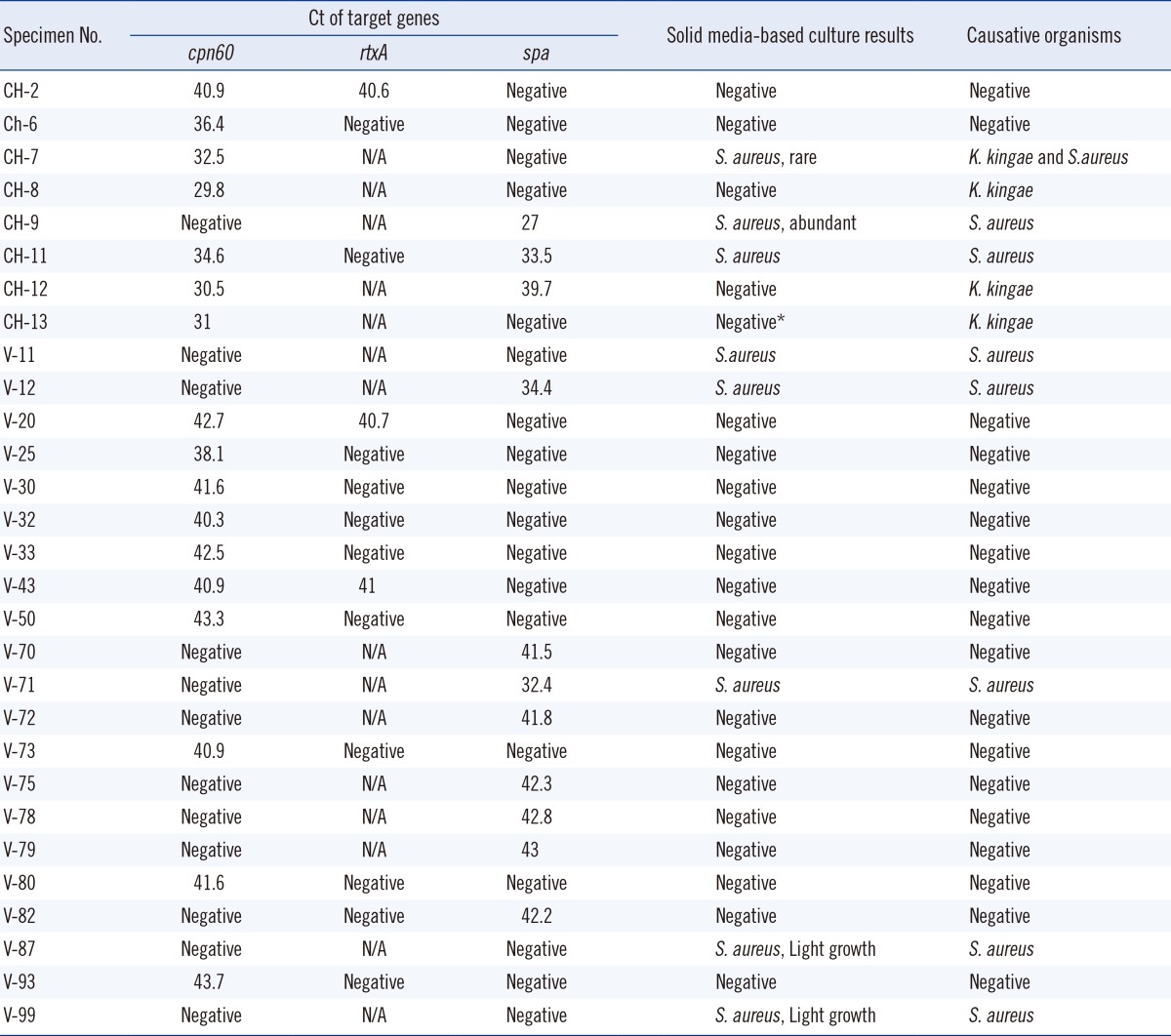
Four of the 117 samples (3.4%) were positive for K. kingae by PCR assay (Table 2). Thirteen samples exhibited weak amplification, with a Ct greater than the cut-off value of 34 (Table 3). In the absence of an appropriate gold standard, additional PCR with rtxA-specific primers was performed on the 13 samples. As these samples also failed to meet the Ct criteria for rtxA PCR, they were deemed negative for K. kingae (Table 3). Prolonged Ct (>cut-off value) might represent non-specific amplification from the host genome or might be due to probe degradation at higher cycles. All K. kingae-positive samples were from pediatric patients (Table 2). Gram staining of SF or direct plating on solid agar-based media did not detect K. kingae in any specimen (Tables 2 and 3). One of the 4 PCR-positive samples was directly inoculated into a blood culture broth (Bactec Peds Plus, aerobic bottle, incubated in the Bactec FX system) (BD, Franklin Lakes, NJ, USA), and under these conditions, K. kingae was isolated. The superiority of PCR over direct plating and the 50% PCR positivity in the 0-5 yr age group are consistent with recent studies from Europe [8]. Inoculation of blood culture broth with SF improves the detection of K. kingae when compared to direct plating [6, 7]. We could not compare the PCR assay with the practice of inoculating blood bottles as only a few samples were subjected to the latter during routine clinical care. However, the superiority of PCR-based detection compared to blood broth inoculation has been demonstrated before [8]. Furthermore, the rapid turn-around time for PCR-based assays is an additional advantage over culture-based detection. Therefore, we recommend PCR-based assays for the detection of K. kingae in pediatric SA, especially in cases with a high index of clinical suspicion.
Four of the 117 samples (3.4%) were positive for S. aureus by PCR (Table 2). Direct plating on agar-based media is the gold standard for the detection of S. aureus. The pathogen grew from all 4 PCR-positive samples (Table 2). Growth was reported as "abundant" for 2 samples and "light" for 1 sample. Direct Gram staining of SF demonstrated gram-positive cocci in 3 out of 4 samples. Four additional samples were culture-positive but PCR-negative (Table 2). These samples were negative for direct Gram staining of SF and demonstrated "light growth" of S. aureus on 3 of the 4 plates (Table 3). Notably, 1 of the 4 PCR-negative culture-positive samples (CH-7) was positive for K. kingae by PCR (Table 3). This sample demonstrated "rare" growth of S. aureus along with some growth of Streptococcus anginosus, suggesting contamination as the probable cause of S. aureus culture positivity in this particular sample. Seven additional samples exhibited weak spa amplification, with Ct >38 (Table 3). However, none were culture-positive for S. aureus. In summary, conventional plating on agar-based media was more sensitive than our PCR assay for the detection of S. aureus in SF, especially with a low pathogen burden. This is not surprising, considering the established limit of detection of this assay. While lowering the cut-off Ct values may enhance sensitivity, we note that none of the PCR-negative (based on Ct ≤38) culture-positive samples demonstrated late Ct positivity (≥38) suggesting an intrinsic limitation of this specific biological matrix (SF). To our knowledge, this is the first report comparing PCR to conventional culture-based methods for detecting S. aureus in SF, and thus, the results will be of interest to laboratories considering molecular techniques for the detection of this pathogen.
References
1. Mathews CJ, Weston VC, Jones A, Field M, Coakley G. Bacterial septic arthritis in adults. Lancet. 2010; 375:846–855. PMID: 20206778.

3. Gafur OA, Copley LAB, Hollmig ST, Browne RH, Thornton LA, Crawford SE. The impact of the current epidemiology of pediatric musculoskeletal infection on evaluation and treatment guidelines. J Pediatr Orthop. 2008; 28:777–785. PMID: 18812907.

4. Yagupsky P. Kingella kingae: from medical rarity to an emerging paediatric pathogen. Lancet Infect Dis. 2004; 4:358–367. PMID: 15172344.
5. Von Essen R, Hölttä A. Improved method of isolating bacteria from joint fluids by the use of blood culture bottles. Ann Rheum Dis. 1986; 45:454–457. PMID: 3524479.

6. Yagupsky P, Dagan R, Howard CW, Einhorn M, Kassis I, Simu A. High prevalence of Kingella kingae in joint fluid from children with septic arthritis revealed by the BACTEC blood culture system. J Clin Microbiol. 1992; 30:1278–1281. PMID: 1583131.
7. Høst B, Schumacher H, Prag J, Arpi M. Isolation of Kingella kingae from synovial fluids using four commercial blood culture bottles. Eur J Clin Microbiol Infect Dis. 2000; 19:608–611. PMID: 11014623.
8. Ilharreborde B, Bidet P, Lorrot M, Even J, Mariani-Kurkdjian P, Liguori S, et al. New real-time PCR-based method for Kingella kingae DNA detection: application to samples collected from 89 children with acute arthritis. J Clin Microbiol. 2009; 47:1837–1841. PMID: 19369442.
9. Lehours P, Freydière AM, Richer O, Burucoa C, Boisset S, Lanotte P, et al. The rtxA toxin gene of Kingella kingae: a pertinent target for molecular diagnosis of osteoarticular infections. J Clin Microbiol. 2011; 49:1245–1250. PMID: 21248099.
10. Chiba N, Murayama SY, Morozumi M, Nakayama E, Okada T, Iwata S, et al. Rapid detection of eight causative pathogens for the diagnosis of bacterial meningitis by real-time PCR. J Infect Chemother. 2009; 15:92–98. PMID: 19396518.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download