Abstract
Background
Detection methods for ABO antibody (Ab) titers vary across laboratories, and the results are different depending on the method used. We aimed to compare titer values using different detection methods for the measurement of ABO Ab titers.
Methods
For ABO Ab detection, pooled group A or B red blood cells (RBCs) were reacted with each of 20 sera from blood groups A, B, or O without dithiothreitol treatment. The room-temperature (RT) incubation technique and the indirect antiglobulin test (IAT) were used in the tube test and gel card test. Flow cytometry (FCM) was performed by using anti-IgM and anti-IgG Abs.
Results
Regardless of the blood groups tested, the FCM assay with anti-IgM showed the highest titer compared to the tube test and gel card test with RT incubation in both. The tube test with IAT showed a higher titer than the gel card test with IAT (Gel-IAT) or FCM with anti-IgG in blood group A and B, while Gel-IAT showed the highest titer relative to the other tests, only for the anti-A Ab in blood group O.
Conclusions
There were significant differences in the titers depending on the detection method used, and each method showed a different detection capacity for each ABO Ab depending on the ABO blood group tested. Therefore, caution should be exercised in interpreting ABO Ab titer results, taking into consideration the detection method used and the blood group.
ABO antigen (Ag) is expressed not only on red blood cells (RBCs), but also on parenchymal organs such as liver, spleen, and kidneys. Therefore, it is necessary to perform ABO antibody (Ab) titration tests for the assessment of prognosis in ABO-incompatible hematopoietic stem cell transplantation or for decision-making in ABO-incompatible kidney transplantation. Despite such practical importance, there are inter-laboratory variations in the measurement of the ABO Ab titer due to the availability of various different techniques and the absence of a standard method.
IgM is the predominant immunoglobulin (Ig) class of anti-A or anti-B Abs produced by group B or A individuals, while IgG is the dominant class of anti-A and anti-B Abs in group O serum. In the hemagglutination (HA) method, the room temperature (RT) incubation technique and the indirect antiglobulin test (IAT) have been interpreted as the methods detecting IgM and IgG, respectively [1, 2, 3]. However, both IgM and IgG of ABO Ab can agglutinate RBCs at RT (20-24℃) or below and efficiently activate the complement at 37℃ [4]. Therefore titers using RT techniques or IAT on dithiothreitol (DTT) untreated samples may be more reflective of the mixed concentration of IgM and IgG of ABO Ab.
The tube test is the most commonly performed method in laboratories. Although in the tube test, a manual reading with the naked eyes is required for grading the agglutination strength and automation of the test is difficult, it is being widely used for general ABO Ab titration in Korea. For the gel card test, one of the column agglutination techniques (CAT), a commercially prepared card with microcolumns containing gel particles is used. The gel card test is more qualitative in grading the strength of agglutination reaction. Additionally, it is less time-consuming and uses smaller volumes of serum and RBCs, and can be used as part of an automated system. Therefore, the number of laboratories using CAT as a method for ABO Ab titration has increased in recent years, despite the need for expensive reagents. Recently, application of flow cytometry (FCM) was suggested as a sensitive ABO Ab detection method [5, 6, 7, 8], and was reported to accurately measure IgM and IgG by the use of isotype-specific Ab. Some studies have reported that FCM produces relatively comparable results to those of the microplate HA assay [5] and CAT [8]. While FCM has been known as a more sensitive method than an agglutination method for Ab detection such as the HLA Ab, the evidence remains too sparse to draw a conclusive evaluation for detecting ABO Ab.
In this study we aimed to compare the ABO Ab titers obtained with the tube test and CAT, and to assess the detection capacity of FCM in comparison with HA.
Serum specimens from apparently healthy adults selected from those who underwent a medical examination at Ajou University Hospital (Suwon, Korea) and from donor RBCs collected from the Korean Red Cross Blood Center were used. These samples were collected from September to December 2012. Aliquots of pooled sera from at least three samples of the same blood group were stored at -70℃ until the ABO Ab titer was measured. Two hundred seventy-four samples were used, and the median age of individuals was 46 yr with a range from 27 to 74 yr Table 1.
In order to reduce the variance of antigen intensity among individuals, the RBC suspension was prepared for titration by pooling three sealed segments of donor blood each preserved with citrate-phosphate-dextrose-adenine-1 (CPDA-1). Each segment of the blood bag was analyzed within 2 weeks from the date of blood collection. This study was approved by the Institutional Review Board of Ajou University Hospital (Med-KSP-12-236).
Serial two-fold dilutions of sera were performed with 0.2 mL of 0.9% normal saline (NS), and a volume of 0.2 mL of sera was added to 10 test tubes. Sera were not treated with DTT. The RBCs were washed three times in NS and a 3% RBC suspension was prepared for the tube test. When RBCs were used in other methods, they were resuspended in NS resulting in a 0.8% RBC suspension for the gel card test and a 1.0% RBC suspension for FCM after the extra-fixation process.
In addition to 50 µL of the group A or B 3% RBC suspension, 100 µL of diluted serum was pipetted into 10 test tubes. Tubes were incubated at RT for 30 min followed by incubation for 30 min at 37℃ with subsequent conversion to the IAT using monospecific anti-IgG (Lorne Laboratories Ltd., Reading, UK). Titer was determined as the highest dilution showing 1+ agglutination [9].
All procedures were performed according to the manufacturer's manual. Twenty-five microliters of serially diluted serum and 50 µL of the prepared group A or B 0.8% RBC suspension were added to the gel card microcolumns. For direct agglutination, the ID-Card NaCl, Enzyme Test, and Cold Agglutinins (Dia-Med AG, Cressier, Switzerland) were used for incubation at RT for 15 min. The ID-Card LISS/Coombs (Dia-Med AG) was used for IAT with incubation at 37℃ for 15 min. The card was centrifuged, and the titer was determined as the highest dilution showing 1+ agglutination.
To prevent hemagglutination or hemolysis, RBC fixation was performed before sensitization. For achieving proper conditions of the fixing reagent, a modified method of Berneman et al. [10] was used to evaluate the effect of glutaraldehyde based on the concentration applied. A 0.025% concentration was selected as the optimal final concentration for fixation (data not shown). It was confirmed that RBCs were not agglutinated under the microscope and in the dot plot of FCM after the Ag-Ab complex were incubated with fluorescent reagent (Fig. 1).
We used a modified version of the method by Stussi et al. [5] and Won [11], to perform a semiquantitative FCM measurement of ABO Ab. First, 500 µL of 3% RBC suspension was fixed with the same volume of 0.05% glutaraldehyde at RT for 5 min. Then, the RBCs were washed three times in phosphate buffered saline (PBS) and resuspended to a final concentration of about 1% (0.1×106/µL). To sensitize RBCs with ABO Ab, 40 µL of fixed RBCs was incubated with 50 µL of the serum in each tube at RT for 30 min and washed three times in PBS. The bound anti-A or anti-B was incubated with fluorescein isothiocyanate (FITC)-labeled Ab for 30 min at RT in the dark, followed by two washes before FCM measurement. In order to detect IgM and IgG, goat anti-human IgM (µ chain specific) (Beckman Coulter, Fullerton, CA, USA) and goat anti-human IgG (γ chain specific) (Beckman Coulter) were used, respectively.
Cytomics FC 500 (Beckman Coulter) with CXP Software (Beckman Coulter) was used for data acquisition and analysis. RBCs were characterized in dot plots by forward/side scatter (FSC/SCC) using a logarithmic scale and a minimum of 10,000 events were collected (Fig. 1). To compare the levels of Ab binding, the geometric mean fluorescence intensity (MFI) ratios were calculated by dividing the MFI of the sera of interest with the MFI of the negative control (O RBC sensitized with AB serum). The cut-off value was calculated on the basis of the 'average MFI ratio + 3SD' [8] of data obtained by measuring 30 negative specimens during 14 days. Specimens used for setting the cut-off value were the following [serum blood group-RBC (number)]: AB-A(6), AB-B(6), AB-AB(1), A-A(6), B-B(5), A-O(3), B-O(3).
Three different methods and two different detection techniques per method were used with the same serum and randomly selected RBCs of the same blood group; they included tube test with RT incubation (Tube-RT), tube test with IAT (Tube-IAT), gel card test with RT incubation (Gel-RT), gel card test with IAT (Gel-IAT), FCM with anti-IgM (FCM-IgM), and FCM with anti-IgG (FCM-IgG). Pooled random group A (or B) RBCs were reacted with 20 sera from blood group B (or A), and pooled random group A RBCs and pooled random group B RBCs were reacted with 20 sera from blood group O, respectively. Although DTT untreated samples were used in our study, RT incubation and IAT have been used to detect the IgM and IgG in HA, respectively. Therefore, titers of Tube-RT and Gel-RT were compared with that of FCM-IgM, and those of Tube-IAT and Gel-IAT were compared with that of FCM-IgG.
To evaluate the detection capacities of FCM and HA, titers of the same specimen were measured by the tube test and gel card test, and then FCM was performed with the serum dilutions of 1) the maximum titer obtained with HA (positive samples of HA, n=20 for each ABO Ab in ABO blood group) and 2) two-fold dilution of the maximum titer (negative samples of HA, n=20 for each ABO Ab in ABO blood group).
The number of titer steps was defined as the number of two-fold dilution for analyzing the titers (i.e., titer step 3 for 1:8 dilution). Statistical analysis was performed by using Microsoft Excel 2007 (Microsoft, Redmond, WA, USA) and SPSS 12.0 for Windows (SPSS, Chicago, IL, USA). The comparison of mean titer steps obtained with the tube test and gel card test was performed by using a paired t-test. The comparison of ABO Ab of FCM and HA using tube test or gel card test was evaluated by using the McNemar test. A P<0.05 was considered statistically significant.
The titer distributions for the tube test and gel card test with two phases (RT and IAT) are described in Fig. 2. The titers of Tube-RT were almost always higher than those of Gel-RT in all the blood groups, with the mean differences in titer steps as follows: 2 (blood group B, P<0.001), 3 (blood group A, P<0.001), and 1 (anti-A and anti-B in blood group O, P<0.001). The differences in the titer steps using the IAT technique, however, varied according to the blood groups. The mean titer steps of Tube-IAT were significantly higher than those of Gel-IAT; only in the blood group A and B were the mean differences in the titer steps the same as those in the titer steps of RT incubation. In blood group O, the mean titer step of Gel-IAT was significantly higher than that of Tube-IAT for anti-A (P<0.01); however, there was no significant difference in the mean titer step for anti-B between the two methods.
The cut-off values of MFI ratios for FCM-IgM and FCM-IgG were 1.48 (=1.01+3×0.16) and 1.83 (=1.05+3×0.26), respectively.
FCM-IgM returned 16-20 positive results (depending on the ABO blood group) in 20 samples that tested positive by HA, but also 13-17 positive results out of 20 samples that tested negative by HA. In blood groups A and B, FCM-IgG returned negative results for all 20 negative samples of HA but also for all 20 samples that tested positive by HA. In the anti-A assay of blood group O, FCM-IgG showed positive results in 9 samples out of 20 samples of HA and in only 3 samples out of 20 samples that tested negative by HA (Table 2).
Because of significant inter-laboratory variations in widely used tube tests for ABO Ab titer measurement, the use of the CAT and FCM methods as an alternative has increased in recent years. Most clinicians however, remain focused only on the target titer without taking into account the differences that may arise in these values due to the choice of the method used. In light of these differences, clinicians should be cautious in interpreting the results of ABO Ab titer, and laboratories should be provided with appropriate information that would warrant a more accurate interpretation of results.
Our results demonstrate that each method or technique including FCM could show different detection capacities for ABO Ab depending on the ABO blood group. While previous studies [5, 8, 12, 13] have pointed out significant differences in titer measurements due to the detection method used, to the best of our knowledge, ours is the first to rigorously demonstrate the dependence of these test results on blood group as well as the method used. Furthermore, given the lack of ABO blood group classification in the previous studies, we were unable to compare our data with the reported data. Moreover, this is the first study comparing the diagnostic performance of FCM with HA including tube test and gel card test.
In our study, FCM-IgM showed higher titers than HA with RT incubation in all of the ABO blood groups, and FCM-IgG showed lower titers than HA with IAT except for the anti-B assay in blood group O samples. FCM has been known as the most valid method for detecting ABO Ab because it can detect not only hemagglutinating or hemolysing ABO Ab but also all ABO Ab binding to the respective ABO Ag expressed on RBCs [5]. However, there is a possibility that higher titers of FCM-IgM compared to those of Tube-RT or Gel-RT, may be due to either the amplification effect of secondary Ab or the highest serum-to-cell ratio among the three methods.
Cho et al. [12] reported that CAT was a more sensitive method than the tube test. However, they used immediate spin technique (IS) for the tube test instead of the more sensitive 30 min RT incubation, as in our study. Furthermore, with CAT there is a possibility that the use of glass beads may show a higher ABO Ab titer than when a gel matrix is used. Moreover, Cho et al. used 'w+' grade as the endpoint instead of '1+' grade as in our study, which may have also affected the results. Also, they used microcolumns using glass beads as the column ingredient, with high serum-to-cell ratio that was more than two times that of ours. Tanabe [7] pointed out that the bead column agglutination method (also used by Cho et al.) shows worse reproducibility with high maximum titer than gel column agglutination method, and also that serum-to-cell ratio is slightly higher in CAT using glass beads. Because serum-to-cell ratios suggested by manufacturer's instructions are different depending on column ingredient, it is important to distinguish between the two methods when comparing target levels of ABO Ab titer.
Some previous studies have reported that the titers obtained with Gel-IAT are comparable to those obtained with Tube-IAT [13]. In our study, however, three methods using sera without DTT treatment showed different detection capabilities of the IgG type of ABO Ab according to the ABO blood group. Therefore, caution should be exercised when interpreting the detection capabilities and selecting methods of the ABO Ab.
Without DTT treatment, the IgM type of ABO Ab that reacts at 37℃ can interfere with the detection of the IgG type of ABO Ab, particularly in tube tests. Tube tests show higher titer in detecting the IgM type of ABO Ab than the gel card test. For the tube tests in this study, the monospecific anti-IgG antibodies used targeted both the heavy and light chains of IgG, where the latter could cross-react with IgA and IgM. In addition to this, the polyspecific antihuman globulin (AHG) used in the gel card test could cross-react with IgA and IgM, as well as with the complement components. Hence, the tube or the gel card test used with the AHG can show a higher titer for the IgG type of ABO Ab compared to FCM that uses an isotype-specific Ab. In this study, it was not possible to perform ABO Ab titration using the gel card test with monospecific anti-IgG, due to its unavailability in Korea. Furthermore, it is not plausible to completely rule out the possibility of false-negative results in FCM, given limited optimization of the protocol used.
Shirey et al. [13] reported that ABO Ab titers measured using AHG are critical for clinical management. The IgM type of ABO Ab can interfere in detection of the IgG type of ABO Ab when specimen without DTT treatment are used, especially in blood groups A and B, which mostly consist of the IgM type of ABO Ab. DTT treatment is needed to measure the exact IgG titration. However, the process for DTT treatment is cumbersome, time consuming, and may destroy IgG [14]. According to the 'uniform procedure' for ABO Ab titration suggested by the College of American Pathologists, the tube test can be converted to the AHG phase after 30 min of RT incubation without DTT treatment [15]. Therefore, if DTT has not been used for titration of the IgG type of ABO Ab, results need to be reported as a measure of 'total Ab', and not 'IgG'. Alternatively, the gel card test is better in detecting the IgG type of ABO Ab, and may be used instead.
CAT is well known for reducing the inter-laboratory variations in ABO Ab titers [2] as well as the turn-around time [13]. However, given the requirement of expensive reagents and the financial limitations under the domestic reimbursement system, the use of CAT as a routine method for measuring the ABO Ab titer is limited in Korea. Previous studies where FCM was used to measure ABO Ab titers, RBCs were fixed with Karnovsky's fixative (glutaraldehyde, 0.05%; formalin, 0.04%; CaCl2 0.00005%; 0.1M cacodylate buffer, 0.0002%; pH=7.4) and washed in PBS containing 6% and 0.6% bovine serum albumin [5]. A recent report presented a relatively simplified protocol of incubation with 0.1% paraformaldehyde for 30 min at 4℃ [8], but they could not measure IgM due to severe agglutination and hemolysis. In this study, we proposed a simpler solution compared to previous studies, using a single glutarldehyde reagent that allows measuring not only IgG but also IgM. Our proposed technique minimizes agglutination and hemolysis of RBCs and allows the measurement of IgM by treating RBC suspension in a short period of time using only a single reagent, glutaraldehyde.
In summary, except for anti-B measurement in blood group O, there were significant differences in the IgM and IgG ABO Ab titers depending on the detection method used. The ABO Ab detection capability for each detection method varied depending on the ABO blood group of the sample; FCM-IgM showed the highest titer compared to Tube-RT or Gel-RT in all of the blood groups. Tube-IAT showed the highest titers in blood groups A and B, while Gel-IAT showed the highest titer only for anti-A detection in group O. These results strongly suggests for caution to be exercised in interpreting the ABO Ab titer, taking the method used into consideration, particularly when making a decision for Ab removal.
Acknowledgments
This study was supported by the Korean Association of Quality Assurance for Clinical Laboratory (KAQACL) Research Fund of 2012-2.
References
1. Tobian AA, Shirey RS, King KE. ABO antibody titer monitoring for incompatible renal transplantation. Transfusion. 2011; 51:454–457. PMID: 21388388.

2. Kumlien G, Wilpert J, Säfwenberg J, Tydén G. Comparing the tube and gel techniques for ABO antibody titration, as performed in three European centers. Transplantation. 2007; 84(12 Suppl):S17–S19. PMID: 18162980.

3. Kobayashi T, Saito K. A series of surveys on assay for anti-A/B antibody by Japanese ABO-incompatible Transplantation Committee. Xenotransplantation. 2006; 13:136–140. PMID: 16623808.

4. Roback JD, editor. Technical Manual. 17th ed. Bethesda: American Association of Blood Banks;2011. p. 369.
5. Stussi G, Huggel K, Lutz HU, Schanz U, Rieben R, Seebach JD. Isotype-specific detection of ABO blood group antibodies using a novel flow cytometric method. Br J Haematol. 2005; 130:954–963. PMID: 16156865.

6. Yung GP, Valli PV, Starke A, Mueller RJ, Fehr T, Cesar-Ozpamir M, et al. Flow cytometric measurement of ABO antibodies in ABO-incompatible living donor kidney transplantation. Transplantation. 2007; 84(12 Suppl):S20–S23. PMID: 18162982.

7. Tanabe K. Interinstitutional variation in the measurement of anti-A/B antibodies: the Japanese ABO-incompatible Transplantation Committee survey. Transplantation. 2007; 84(12 Suppl):S13–S16. PMID: 18162979.

8. Won DI, Kim BC. Optimized flow cytometry to measure anti-ABO immunoglobulin G. Lab Medicine. 2012; 43:281–290.

9. Roback JD, editor. Technical Manual. 17th ed. Bethesda: American Association of Blood Banks;2011. p. 907.
10. Berneman ZN, van Bockstaele DR, Uyttenbroeck WM, Van Zaelen C, Cole-Dergent J, Muylle L, et al. Flow-cytometric analysis of erythrocytic blood group A antigen density profile. Vox Sang. 1991; 61:265–274. PMID: 1776244.

11. Won DI. Comparison method of reactivity of anti-human immunoglobulin reagents for flow cytometry. Korean J Lab Med. 2003; 23:214–219.
12. Cho CH, Kim HN, Yun SG, Choi GR, Choi JY, Kim JS, et al. Evaluation of ABO antibody titration using tube and column agglutination techniques. Lab Med Online. 2011; 1:57–63.

13. Shirey RS, Cai W, Montgomery RA, Chhibber V, Ness PM, King KE. Streamlining ABO antibody titrations for monitoring ABO-incompatible kidney transplants. Transfusion. 2010; 50:631–634. PMID: 19906036.
14. Knight RC. Measuring IgG anti-A/B titres using dithiothreitol (DTT). J Clin Pathol. 1978; 31:283–287. PMID: 641203.

15. AuBuchon JP, de Wildt-Eggen J, Dumont LJ. Reducing the variation in performance of antibody titrations. Vox Sang. 2008; 95:57–65. PMID: 18479347.

Fig. 1
Comparison of the effects of fixation of red blood cells (RBCs) on cell surface. Each sample was examined in the unfixed state (A) and after fixation with 0.05% glutaraldehyde (B). The same RBCs were used in both dot plots; Group B RBCs were reacted with A serum at room temperature and then incubated with fluorescein isothiocyanate (FITC)-labeled goat anti-human IgM.
Abbreviations: SS, side scatter; FS, forward scatter.
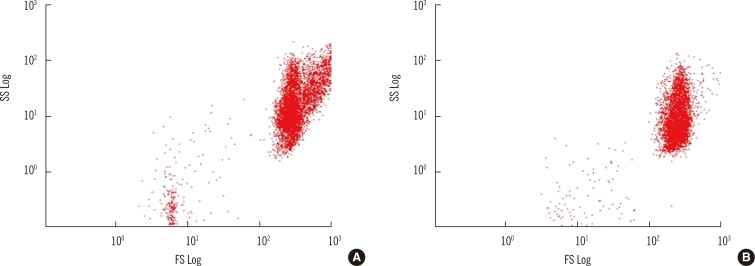
Fig. 2
Comparison of ABO antibody titers between the tube test and the gel card test according to the blood group.
*Mean difference in titer steps vs. gel card test; †P<0.001 vs. Gel card test; ‡P<0.01 vs. Gel card test.
Abbreviations: Tube-RT, tube test with room temperature incubation method; Tube-IAT, tube test with indirect antiglobulin test; Gel-RT, gel card test with room temperature incubation method; Gel-IAT, gel card test with indirect antiglobulin test.
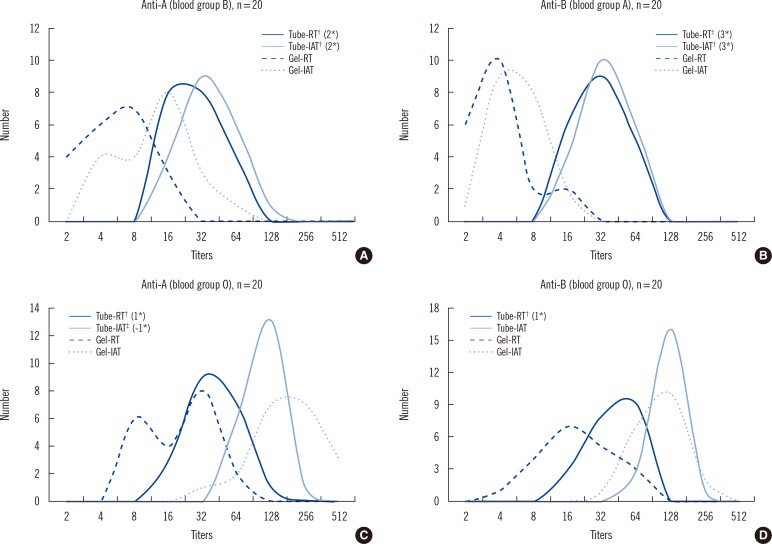
Table 2
Comparison of ABO antibody titer measured by flow cytometry and hemagglutination at the dilution of maximum titer and two-fold dilution of the titer in HA
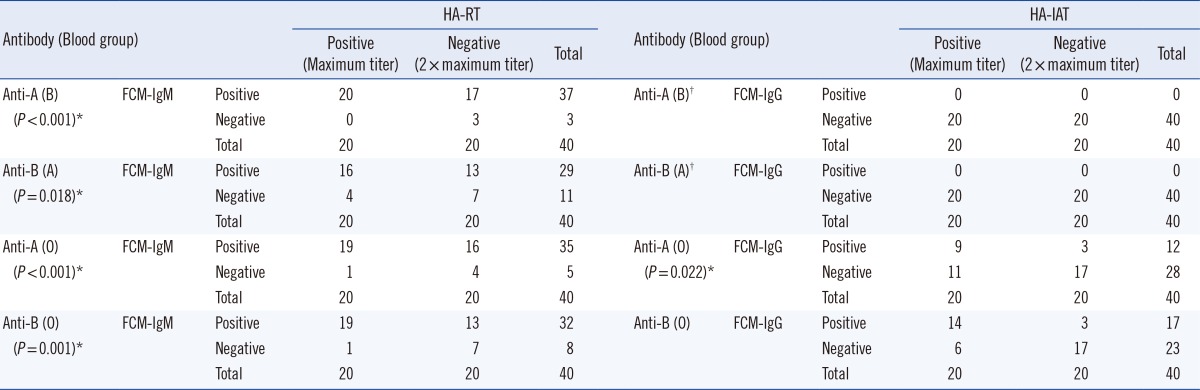
*P value for McNemar test; †Statistical analysis is not available.
Abbreviations: FCM-IgM, flow cytometry with anti-IgM; FCM-IgG, flow cytometry with anti-IgG; Hemagglutination-RT, tube test or gel card test with room temperature incubation; Hemagglutination-IAT, tube test or gel card test with indirect antiglobulin test.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download