Abstract
Background
Early laboratory detection of Mycobacterium tuberculosis is crucial for controlling tuberculosis. We developed a hydrogel mycobacterial culture method that retains the advantages of both solid and liquid methods in terms of speed, cost, and efficiency.
Methods
Mycobacterium bovis bacillus Calmette-Guérin (BCG) suspensions and 200 acid-fast bacilli (AFB)-positive clinical specimens were inoculated in Middlebrook 7H9 liquid media (Becton-Dickinson and Company, USA) and mixed with 75 µL of 9-fluorenylmethoxycarbonyl (Fmoc)-Phe-Phe-OH hydrogel stock solution in an Eppendorf tube just before culture incubation. The mixtures were cultured at 37℃ for as long as 14 days to monitor culture status.
Results
The number of M. bovis BCG increased with time. For 200 AFB smear-positive specimens, 155 of 158 conventional culture-positive specimens and 4 culture-negative or contaminated specimens yielded positive cultures within 14 days. For 128 specimens positive with the liquid culture method, the time to positive culture using the hydrogel method (mean, 12.6 days; range, 7 to 14 days) was significantly shorter than that for conventional liquid culture (mean, 16.2 days; range, 6 to 31 days; P<0.0001).
Tuberculosis (TB) is an important cause of morbidity and death, particularly in third-world countries [1]. Culturing is essential for the accurate diagnosis of TB and other mycobacterial infections and for drug susceptibility testing, but it takes considerable time [2]. Culture also enables the detection of a greater number of TB cases, especially when the acid-fast bacillus (AFB) smear is negative. However, mycobacterial cultures on solid media, which are commonly used in Korea and countries with high TB burdens, usually require 3 to 8 weeks [3]. Liquid culture systems could shorten the incubation, but commercially available systems require expensive consumables and equipment, making them difficult to apply in countries, in which TB is a significant problem.
Gel culture methods have recently been adopted for 3-dimensional cell cultures, in which cells are encapsulated in microporous, nanofibrous and hydrogel scaffolds [4-6]. Cells can maintain a 3-dimensional structure within the spaces of the scaffolds and use a sufficient amount of nutrients dissolved in the liquid inside. These methods are not commonly used for ordinary microorganism cultures, because they have less merit than conventional growth media methods, which enable rapid growth of most microbes. Rapidly growing non-fastidious bacteria can be cultivated in simple liquid or solid media. However, mycobacterial culture is different. It requires a long time, and obtaining results using liquid culture can save several days or weeks over that required using solid culture.
We believe that applying a gel culture method to mycobacteria might provide rapid and efficient results in a small volume of medium at a reasonable price. Therefore, the current study aimed to develop a hydrogel scaffold culture system that rapidly, efficiently, and economically detects mycobacteria in clinical specimens.
Hydrogel culture stock solution was prepared by adding 100 mg of fluorenylmethoxycarbonyl (Fmoc)-Phe-Phe-OH (Bachem, Bubendorf, Switzerland) to 1 mL of dimethylsulfoxide (DMSO, Junsei Chemical, Tokyo, Japan) and sterilizing the mixture via filtration.
The Mycobacterium bovis bacillus Calmette-Guérin (BCG) strain was provided by the Korean Institute of TB. It was cultured in oleic acid-albumin-dextrose-catalase complex (OADC)-enriched Middlebrook 7H9 broth medium (DIFCO Middlebrook 7H9 broth; Becton-Dickinson and Company, Franklin Lakes, NJ, USA) in a 37℃ shaking incubator for as long as 10 days until the medium became turbid. The cultured medium was diluted with Middlebrook 7H9 broth to adjust the absorbance to 0.3 at 540 nm. This diluent was considered to have 1×108 organisms/mL [7, 8] and was designated the ×1 inoculum. Both 102-fold and 104-fold dilutions of ×1 cultures were prepared. Next, 75 mL of ×1, ×102, and ×104 dilutions were transferred into Eppendorf tubes, and 75 mL of the hydrogel stock solution was added to each tube. Five tubes were prepared for each concentration in 1 event, and the events were repeated twice.
As soon as the stock solution was added, the culture medium solidified to a gel at room temperature. The Eppendorf tubes were incubated in a 37℃ room air incubator for as long as 14 days. For checking growth status, at the beginning of incubation (day 0) and after 4, 7, 10, and 14 days of incubation, 1 tube for each dilution was homogenized (gel reversal), smeared onto a glass slide, stained with auramine-rhodamine, and observed under a fluorescence microscope (Nikon Microscope ECLIPSE 80i, Tokyo, Japan). The gel homogenization procedure is described below.
Two hundred smear-positive specimens were selected from clinical sputum specimens submitted to the mycobacteriology laboratory at Pusan National University Yangsan Hospital during June and December 2010 (Table 1). Conventional decontamination and concentration were carried out for all specimens. Briefly, specimens were mixed with the same volume of 2% NaOH and 1% N-acetyl-L-cysteine (NALC). After 15 min of incubation, phosphate-buffered saline was added and the mixture was centrifuged at 3,000×g for 15 min. The supernatant liquid was withdrawn, and the remaining 500 mL was used.
AFB staining using a fluorescence microscope, liquid culture using an MGIT 960 system (Becton Dickinson Microbiology Systems, Sparks, MD, USA), and solid culture using 3% Ogawa medium (Asan Pharmaceutical, Seoul, Korea) were simultaneously performed. The staining grade was recorded from a trace to 4+ according to the U.S. Centers for Disease Control and Prevention scale. Next, 60 mL of OADC-enriched Middlebrook 7H9 broth and 15 mL of processed clinical specimen were added to the Eppendorf tube followed by 75 mL of hydrogel stock solution, and the culture medium solidified spontaneously into a gel form. Five tubes were prepared for each of the 200 clinical specimens and were incubated in a 37℃ room air incubator for as long as 14 days. The process to check growth status was the same as that outlined above.
The growth of mycobacteria was confirmed with AFB staining after hydrogel reversal. The hydrogel culture results of the clinical specimens were compared with the conventional liquid and solid cultures. Only the hydrogel and liquid culture results were used for the comparison of the time to detection of positive culture results.
Homogenization or reversal of the hydrogel to a liquid state is required to stain each cultured hydrogel medium. To accomplish reversal, we added 600 mL of 1N HCl to each tube of cultured hydrogel. After liquefying the hydrogel, we transferred it to new Eppendorf tubes. Each tube was centrifuged for 1.5 min at 2,000 rpm. After centrifugation, the gel sank to the bottom of the tube, and the supernatant liquid obtained contained the bacilli. The liquid was subjected to AFB staining.
The chi-square test was performed to compare the frequency data. The agreement between methods was tested using the Kappa statistics. Kappa results were interpreted as follows: values ≤0 as indicating less than chance agreement; 0.01-0.20 as slight agreement; 0.21-0.40 as fair; 0.41-0.60 as moderate; 0.61-0.80 as substantial; and 0.81-1.00 as almost perfect agreement [9]. The means of 2 groups were compared using a non-parametric test such as the Mann-Whitney U test. Correlation between hydrogel culture and AFB staining grades was assessed with the Spearman ρ test. P value <0.05 was considered significant.
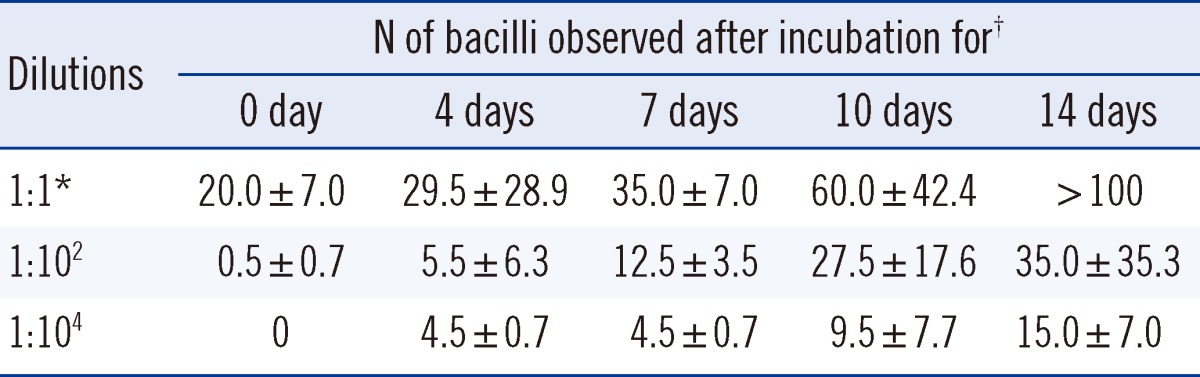
On the day of inoculation of the M. bovis BCG strain, AFB were not detected under the fluorescence microscope in the hydrogel to which a ×104-fold dilution of bacterial suspension had been added, but more than 10 AFB were observed in the ×1-fold dilution of hydrogel. Over time, the numbers of M. bovis observed on AFB staining increased in hydrogels for all concentrations of diluents inoculated (Table 2). Hydrogel cultures with the ×104 suspension, which may contain 1×104 bacilli/mL, yielded positive results by day 4 of incubation.
Among the 200 selected smear-positive clinical specimens, 158 yielded positive cultures for Mycobacterium tuberculosis (MTB) or non-tuberculous mycobacteria (NTM), and the remaining 42 specimens were culture negative or contaminated by conventional mycobacterial culture (see Table 1).
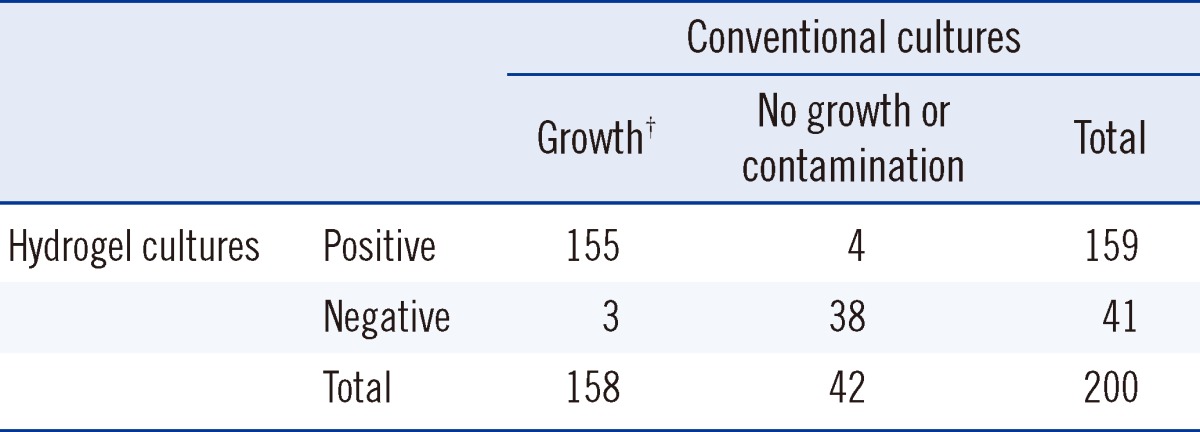
Table 3 shows a comparison of the conventional and hydrogel cultures. Among 158 specimens positive by conventional methods, 155 (98.1%) were positive by hydrogel culture. Growth occurred by the hydrogel method in 4 specimens showing no growth or contamination by conventional methods. Agreement between conventional and hydrogel cultures was nearly perfect (kappa=0.894, 95% confidence interval [CI] 0.816-0.971).
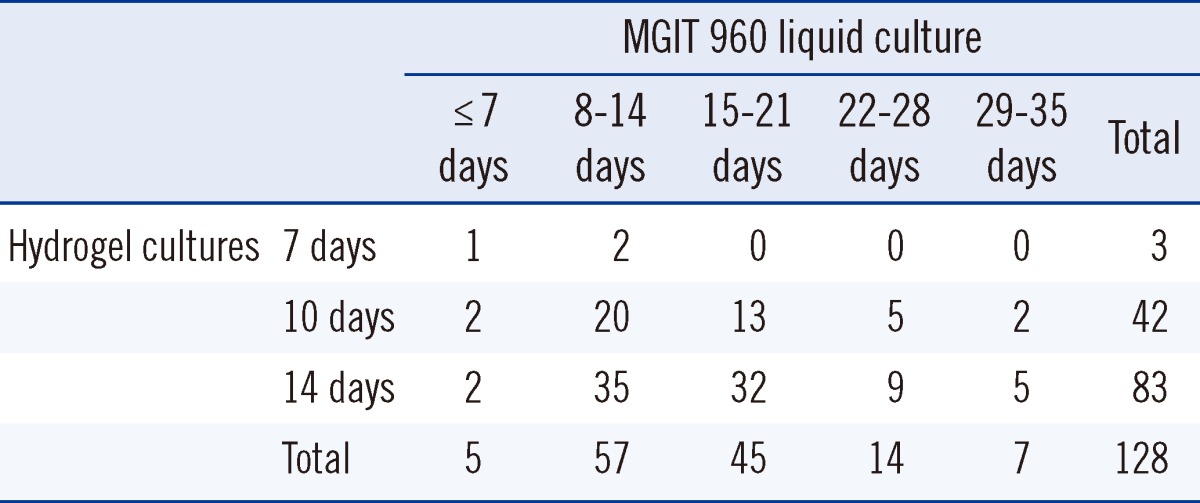
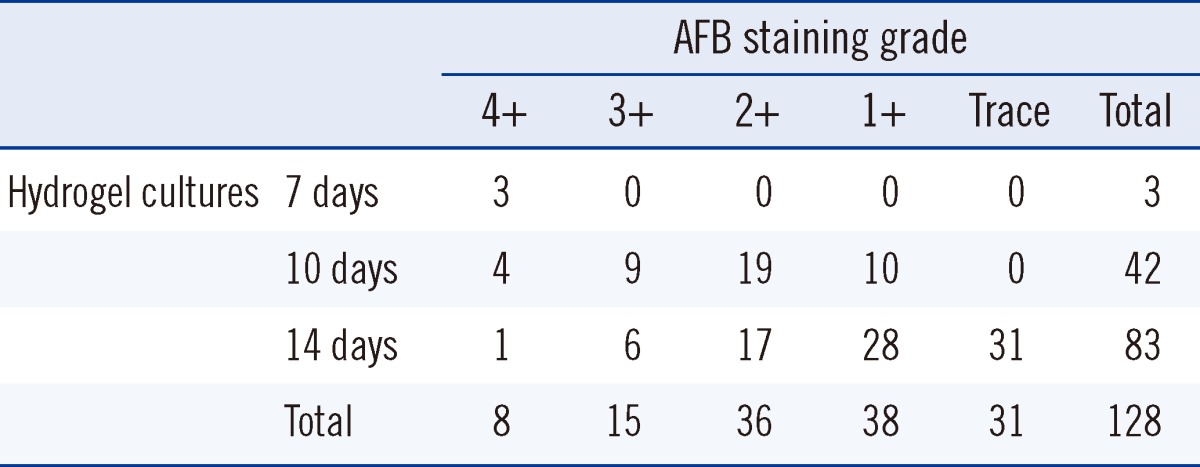
The turnaround time from inoculation to positive culture for the 128 specimens positive by liquid culture has been compared to that for the hydrogel and liquid culture methods in Table 4. Conventional cultures took 6 to 31 days (mean, 16.2 days). Sixty-two (48.4%) of the conventional cultures were positive in 14 days or less, but all positive hydrogel cultures took 7 to 14 days (mean, 12.6 days). The hydrogel culture thus was significantly faster than conventional culture (P<0.0001). Table 5 shows the time to hydrogel culture results compared with AFB staining grades. The culture time of hydrogel culture was inversely correlated with AFB staining grades (Spearman ρ=-0.529, P<0.0001).
TB is well controlled by drugs; therefore, strategies to detect TB early and improve patient compliance with therapy are pivotal in TB control. We tried to find a gel culture method that did not reduce the viability of mycobacteria and was inexpensive and easy to handle. After a search for the simplest possible peptide system that can self-assemble, we found that the combination of dipeptides modified with aromatic stacking ligands forms nanometer-sized fibers when exposed to physiological conditions [10]. Among several self-assembling Fmoc peptides, Fmoc-Phe-Phe-OH compounds were selected because of the wide range of weight % values that gives rise to self-supporting gels and thick fiber diameter (56 nm) that trap mycobacteria within the scaffold of the gel [11]. Therefore, in the current study, we applied the hydrogel culture method for mycobacteria using Fmoc-Phe-Phe-OH, which self-assembles when mixed with a liquid culture medium at room temperature. This self-assembling property of Fmoc-Phe-Phe-OH embedded the mycobacteria inside the gel without damage. The scaffold structures of the gel also enable a supply of oxygen for the mycobacteria within the gel and trap the mycobacteria produced by division in a small area so that a few mycobacteria can be detected easily. Furthermore, Fmoc-Phe-Phe-OH converts easily into a liquid, which provides ease of specimen handling. In this study, we used acidification for gel reversal, but alkalization or simple pressing of the gel could be used for the same purpose. The reversal method should be selected depending on the requirements for further studies, and acidification was the best method for AFB staining.
This study demonstrated that an M. bovis BCG suspension of 104 bacilli/mL turns culture positive in 4 to 7 days. We used this strain first to evaluate the performance of the hydrogel culture system owing to the safety issues accompanying MTB. Therefore, this result could not be directly extrapolated to MTB cultures or other mycobacterial cultures using clinical specimens. To overcome this problem, we applied our method to AFB smear-positive selected sputum specimens and observed an excellent culture result within a shorter time than that required with conventional culture. The results indicated that the scaffold hydrogel culture system has the potential to support mycobacterial growth in a timely manner.
Another problem to be addressed is the best mixture of the dipeptide and liquid medium for forming a hydrogel scaffold structure. DMSO is the most suitable reagent for dissolving Fmoc-Phe-Phe-OH. However, it is cytotoxic, and therefore we sought to minimize the amount of DMSO required to create the hydrogel. However, when the amount was reduced too much, dipeptide and OADC-enriched Middlebrook 7H9 broth culture medium did not fuse equally, and therefore, a gel retaining the appropriate scaffold size was difficult to prepare. To determine the best conditions for the hydrogel system, we evaluated various combinations of the amount of the dipeptide, DMSO, and Middlebrook 7H9 broth medium (data not shown). We decided to use hydrogel stock solution at 100 mg/mL and a mixture of the same 75 mL of stock solution and specimen in an Eppendorf tube for culture.
The clinical applicability of the current method is important. Insufficiently rapid MTB growth and the small number of mycobacteria in most clinical specimens are obstacles to early TB detection by culture. However, our data demonstrated that the extent and speed of mycobacterial growth are superior in the current hydrogel culture compared with those in conventional solid cultures. Almost all culture-positive specimens and a few negative or contaminated specimens by conventional culture turned positive in our hydrogel system, indicating that our method is more sensitive or at least not less sensitive than conventional cultures. Furthermore, the growth of mycobacteria in hydrogel culture was much faster than that in solid media. One study has shown that MTB culture usually requires 14-20 days in commercial liquid systems [12]. Therefore, we believe that our method is competitive with this culture method in this respect.
One limitation of our system is that the detection of mycobacterial growth relies on AFB smears after gel homogenization. Observing colonies is easy in solid cultures. Automatic signals are emitted in commercial liquid culture systems, even though the true growth of mycobacteria should be verified by AFB staining. However, we blindly picked culture tubes to homogenize and stain; therefore, we had to prepare several tubes for the culture of each specimen. Eppendorf tubes are conveniently small, and the total volume required is also small; therefore, we could assume that the results were positive depending on the initial AFB smear positivity.
Another limitation of the current method is that after homogenization, the bacilli might not be viable owing to treatment with hydrochloride, which means that the cultured bacilli cannot be used for phenotypic identification and drug susceptibility testing. The species can be identified using PCR or other molecular methods. However, drug susceptibility testing is still crucial in anti-tuberculous treatment, especially in cases of treatment failure. Therefore, we need to establish other methods for homogenization of the gel and retrieval of live bacilli. In the current study, we did not consider the mycobacterial species -i.e., MTB or NTM-when analyzing the culture recovery rate and time to positive culture. However, almost all isolates were recovered from clinical isolates regardless of the species; therefore, we do not believe that this method is limited only to the recovery of specific mycobacterial species. A study on the differences in hydrogel culture for detecting MTB or NTM would be an insightful subject for future research. In conclusion, we demonstrated that the hydrogel scaffold culture system is useful for timely, economical, and efficient detection of mycobacteria in clinical specimens.
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (2009-0066265).
References
1. World Health Organization. Global tuberculosis report 2012. Publication No. WHO/HTM/TB/2012.6. Geneva: World Health Organization;2012. http://www.who.int/tb/publications/global_report/en/index.html.
2. World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis-emergency update 2008 (WHO/HTM/TB/2008.402). Geneva: World Health Organization;2008.
3. Chang CL, Park TS, Kim MN, Lee NY, Lee HJ, Suh JT. Survey on changes in mycobacterial testing practices in Korean Laboratories. Korean J Clin Microbiol. 2001; 4:108–114.
4. Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci USA. 2003; 100:12741–12746. PMID: 14561891.

5. Semino CE, Merok JR, Crane GG, Panagiotakos G, Zhang S. Functional differentiation of hepatocyte-like spheroid structures from putative liver progenitor cells in three-dimensional peptide scaffolds. Differentiation. 2003; 71:262–270. PMID: 12823227.

6. Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009; 103:655–663. PMID: 19472329.

7. Bollela VR, Sato DN, Fonseca BA. McFarland nephelometer as a simple method to estimate the sensitivity of the polymerase chain reaction using Mycobacterium tuberculosis as a research tool. Braz J Med Biol Res. 1999; 32:1073–1076. PMID: 10464381.
8. Miyamoto J, Koga H, Kohno S, Tashiro T, Hara K. New drug susceptibility test for Mycobacterium tuberculosis using the hybridization protection assay. J Clin Microbiol. 1996; 34:1323–1326. PMID: 8727932.
9. Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005; 37:360–363. PMID: 15883903.
10. Jayawarna V, Smith A, Gough JE, Ulijn RV. Three-dimensional cell culture of chondrocytes on modified di-phenylalanine scaffolds. Biochem Soc Trans. 2007; 35(Pt 3):535–537. PMID: 17511646.

11. Jayawarna V, Ali M, Jowitt TA, Miller AF, Saiani A, Gough JE, et al. Nanostructured hydrogels for three-dimensional cell culture through self-assembly of fluorenylmethoxycarbonyl-dipeptides. Adv Mater. 2006; 18:611–614.

12. Koh WJ, Ko Y, Kim CK, Park KS, Lee NY. Rapid diagnosis of tuberculosis and multidrug resistance using a MGIT 960 system. Ann Lab Med. 2012; 32:264–269. PMID: 22779067.

Table 1
Acid-fast bacillus staining and culture results of 200 selected clinical specimens submitted for mycobacterial evaluation
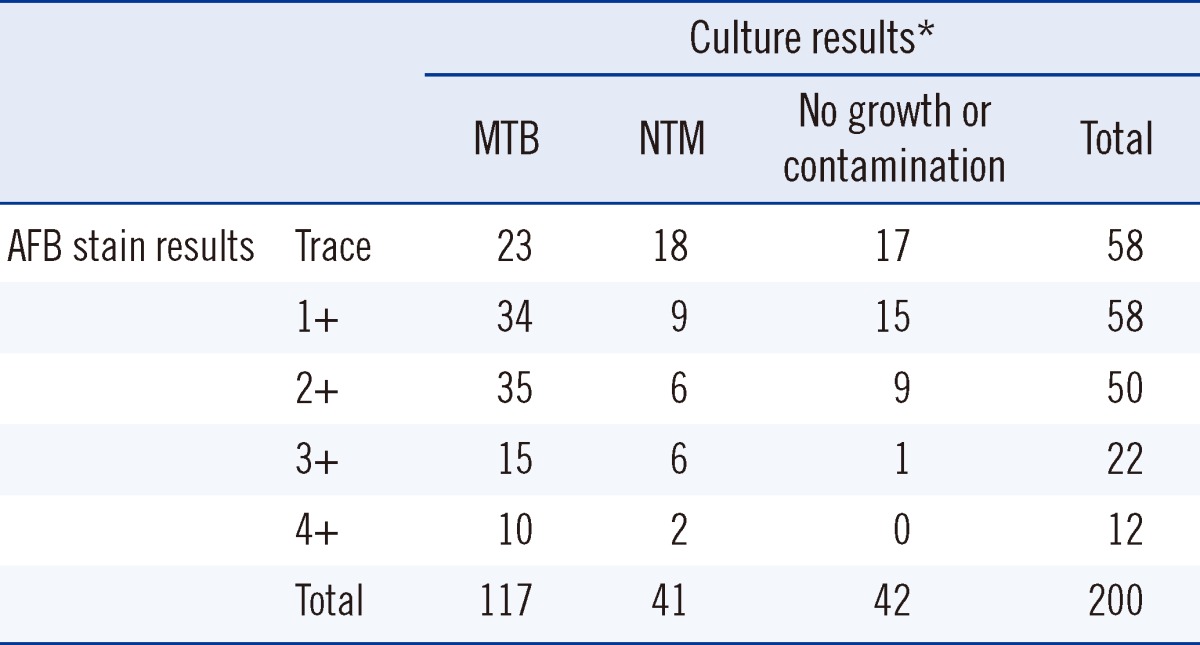
Table 2
Number of acid-fast bacilli observed under the fluorescence microscope after culture of Mycobacterium bovis BCG-suspended medium in the hydrogel culture system




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download