INTRODUCTION
METHODS
1. Study population
2. ABO antibody titration
1) Immediate spin (IS) tube method
2) AHG tube method
3) Column agglutination technique without DTT (CAT without DTT) treatment
4) Column agglutination technique with DTT (CAT with DTT) treatment
3. Statistics
RESULTS
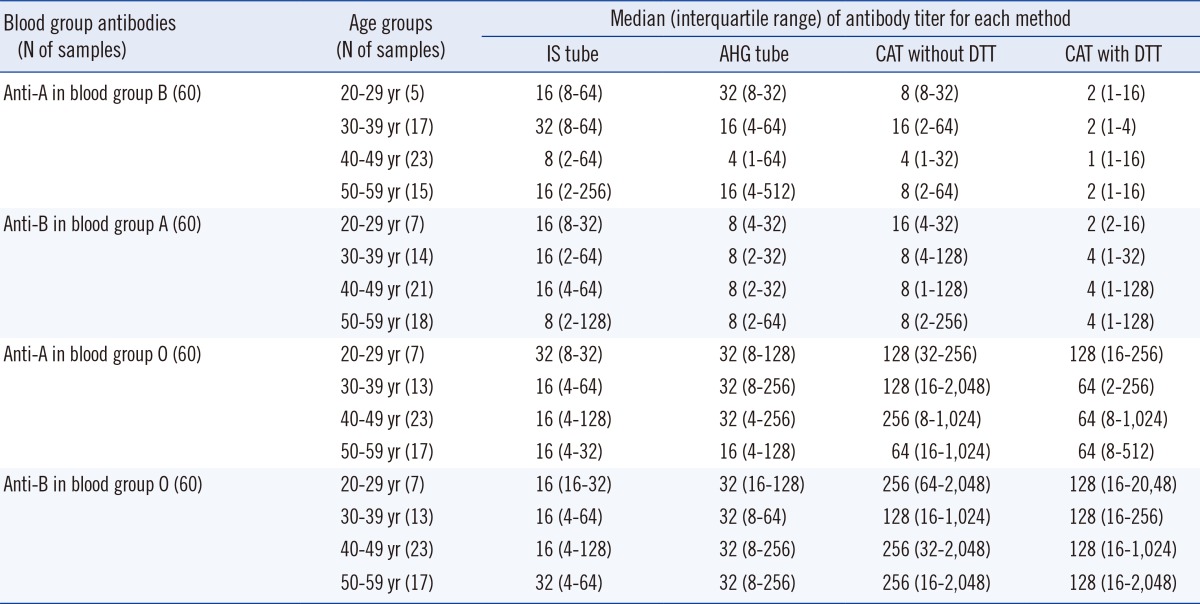
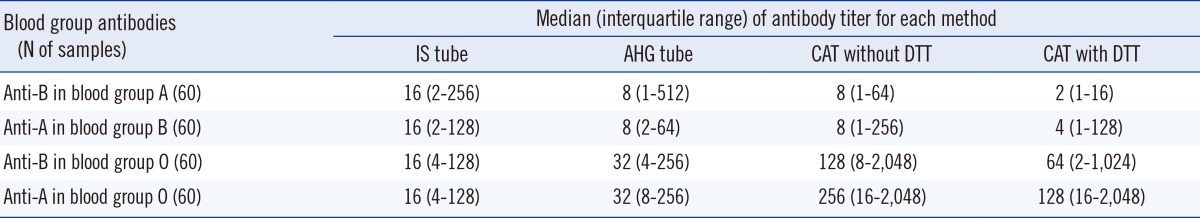
1. Distribution of antibody titers according to age and gender
Table 2

*The titers measured by the IS tube method were higher in females than in males (P=0.005, Mann-Whitney test), but titers by other methods showed no statistical differences between genders.
Abbreviations: IS, immediate spin; AHG, anti-human globulin; CAT, column agglutination technique; DTT, dithiothreitol.
2. Comparison of antibody titers between the IS tube and AHG tube methods
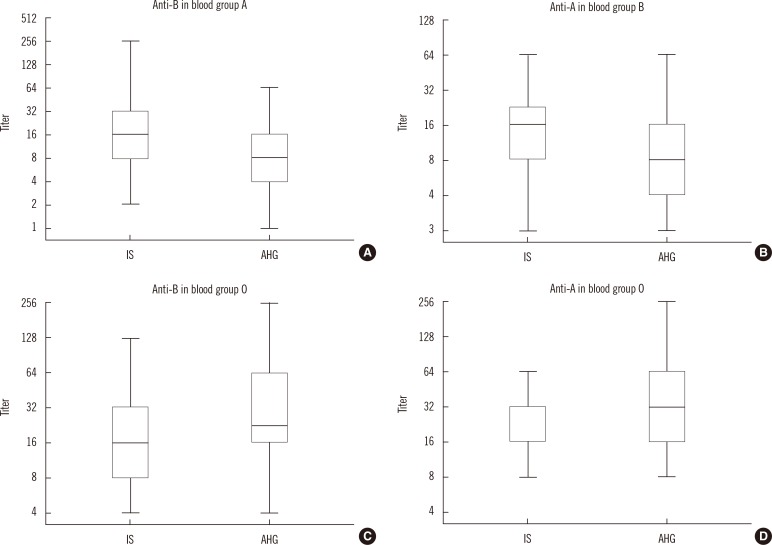 | Fig. 1Comparison of antibody titers obtained using the IS tube and AHG tube methods. The horizontal lines in the middle of each box, the top and bottom borders of each box, and whiskers above and the below the boxes represent median values, interquartile ranges, and error bars, respectively. (A) anti-B in blood group A. (B) anti-A in blood group B. (C) anti-B in blood group O. (D) anti-A in blood group O individuals.
Abbreviations: IS, immediate spin; AHG, anti-human globulin.
|
3. Comparison of antibody titers between AHG tube and CAT without DTT methods
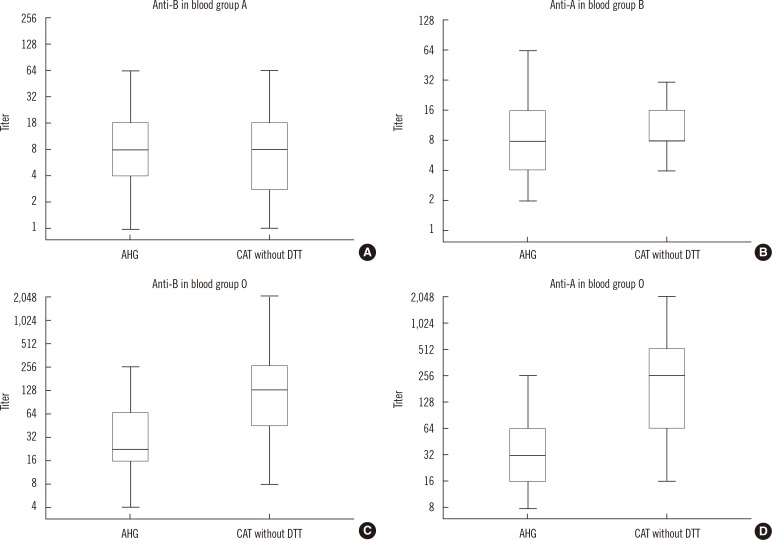 | Fig. 2Comparison of antibody titers obtained using the AHG tube and CAT without DTT methods. The horizontal lines in the middle of each box, the top and bottom borders of each box, and whiskers above and the below the boxes represent median values, interquartile ranges, and error bars, respectively. (A) anti-B in group A. (B) anti-A in group B. (C) anti-B in group O. (D) anti-A in group O individuals.
Abbreviations: AHG, anti-human globulin; CAT, column agglutination technique; DTT, dithiothreitol.
|
4. Comparison of antibody titers obtained using CAT with and without DTT methods
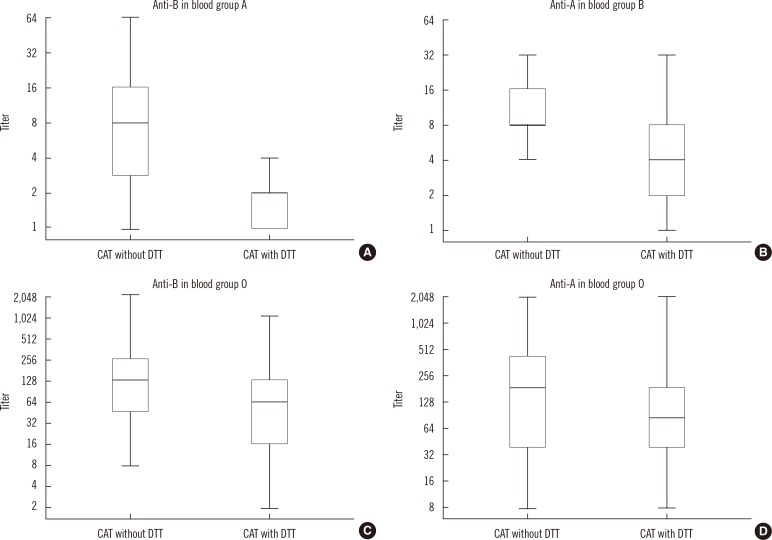 | Fig. 3Comparison of antibody titers obtained using CAT with and without DTT methods. The horizontal lines in the middle of each box, the top and bottom borders of each box, and whiskers above and the below the boxes represent median values, interquartile ranges, and error bars, respectively. (A) anti-B in group A. (B) anti-A in group B. (C) anti-B in group O. (D) anti-A in group O individuals.
Abbreviations: CAT, column agglutination technique; DTT, dithiothreitol.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download