INTRODUCTION
Neuroblastic tumors are embryonal tumors that form around sympathetic nerve ganglions. The relative proportion of Schwann cells and neuroblasts distinguishes stroma-rich tumors such as ganglioneuromas from stroma-poor tumors such as neuroblastomas (NB). Ganglioneuroma is the most benign type of neuroblastic tumor and is characterized by numerous Schwann cells with dispersed maturing ganglionic cells. NB is the most malignant tumor type and consists primarily of neuroblastic cells with a low proportion of Schwann cells [
1,
2].
NBs can be distinguished according to the degree of differentiation of the neuroblastic cells (poorly differentiated, undifferentiated, or differentiating). Poorly differentiated NB is characterized by the presence of small round blue cells with high nuclear-to-cytoplasmic ratios and obvious neurite extensions. Undifferentiated NB shows similar characteristics but lacks neurite outgrowths. Finally, differentiating NB is composed of at least 5% differentiating neuroblastic cells that exhibit synchronous maturation of the cytoplasm, nucleus, and neurofibrils [
1,
2].
A marked variability exists in the clinical behavior of NB [
3]. Approximately 60% of patients with clinically diagnosed NB have stage 4 disease, which is associated with a very poor prognosis. In stage 4S disease (defined as age <1 yr with a localized primary tumor defined as stage 1 or 2, with dissemination limited only to the liver, skin, or bone marrow [BM] with less than 10% of BM nucleated cells are tumor cells) or International Neuroblastoma Risk Group stage MS (defined as metastatic disease 'special', where MS is equivalent to stage 4S), spontaneous maturation and regression without treatment is a well-established phenomenon that improves prognosis and increases survival rates [
4,
5]. This phenomenon is known to occur exclusively in infants younger than 1 yr with a localized primary tumor [
6-
9].
A similar phenomenon was reported in a single previous study, in which spontaneous regression of stage 4 NB was observed in patients with multiple metastases of the brain, scalp, and skeleton [
10]. However, in that report, stage 4 was assessed based only on the multiple metastases and not on the BM metastasis. Therefore, it is likely that the presence of differentiating neuroblasts in the BM of patients with stage 4 NB has not yet been reported. Because retinoic acid is used as a differentiating agent in therapeutic regimens, differentiating neuroblasts are expected to be observed in BM after chemotherapy in some cases. Therefore, the prognostic impact of the presence of differentiating neuroblasts in BM needs to be investigated.
In the present study, we aimed to identify the prognostic impact of the presence of differentiating neuroblasts in BM both at diagnosis and after chemotherapy in patients with BM metastatic NB (stage 4). To determine the clinical significance of differentiation, we compared histology and laboratory data along with overall survival (OS) with regard to the differentiation status of neuroblasts in BM at diagnosis and after chemotherapy.
RESULTS
1. Clinical and laboratory characteristics of patients with BM metastatic NB
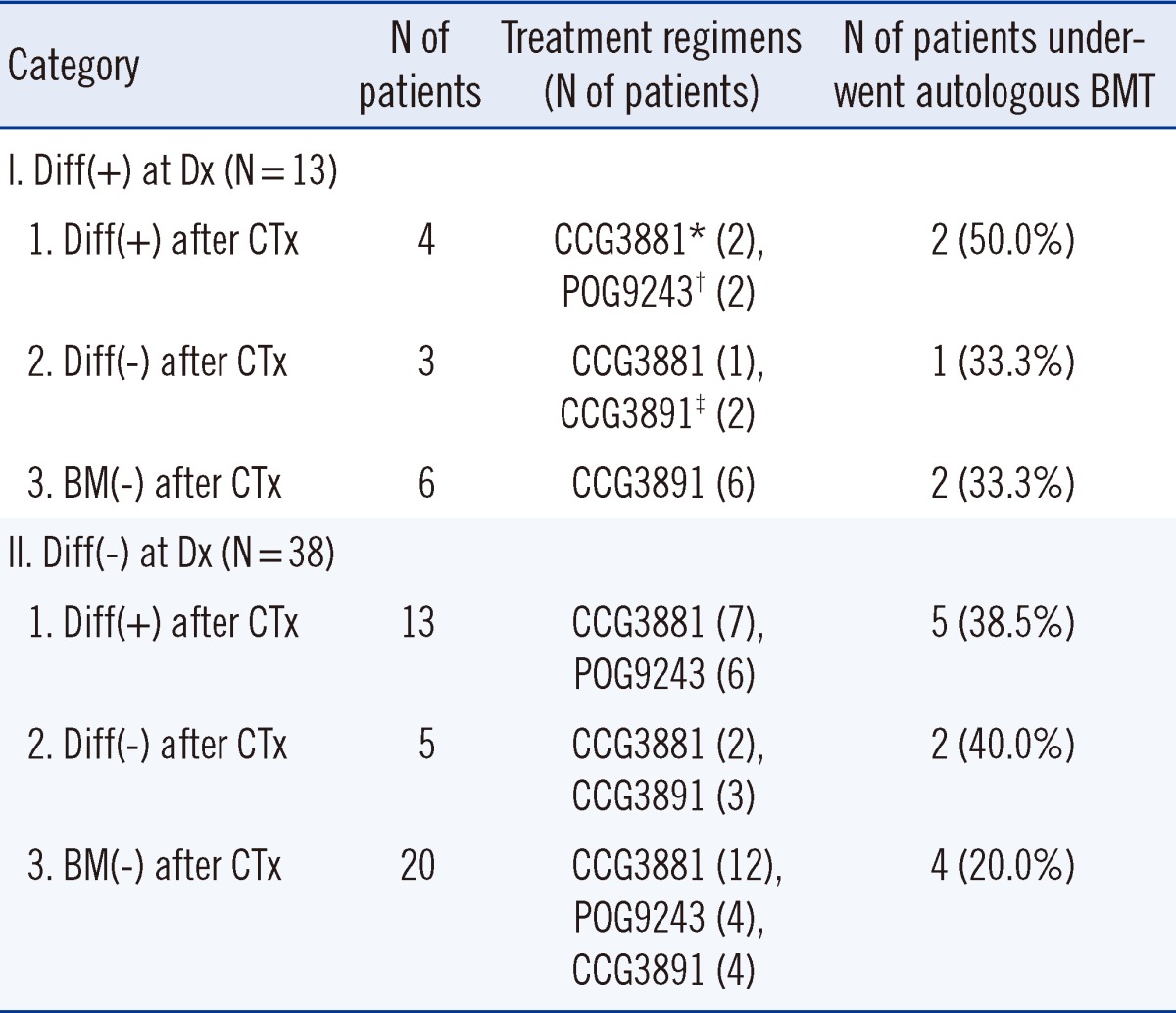
Fig. 1 shows examples of differentiating neuroblasts in BM after conventional chemotherapy. Of the 51 patients with BM metastatic NB, 13 (25.5%) exhibited differentiating neuroblasts in BM at diagnosis. Of these 13 patients, differentiating neuroblasts were detected in 4 patients but not in 3 patients and no evidence of BM metastasis was observed in 6 patients after chemotherapy. The remaining 38 patients (74.5%) did not exhibit differentiating neuroblasts in BM at diagnosis. Of these 38 patients, differentiating neuroblasts were detected in 13 patients but not in 5 patients and no evidence of BM metastasis was observed in 20 patients after chemotherapy.
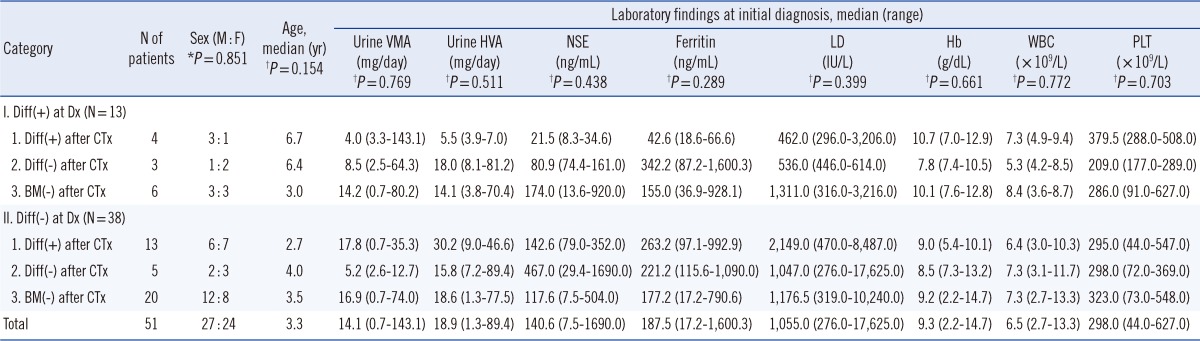
The patient groups did not differ with regard to gender (
P=0.851), age (
P=0.154), VMA (
P=0.769), HVA (
P=0.511), NSE (
P=0.438), ferritin (
P=0.289), LD (
P=0.399), or blood cell counts (hemoglobin,
P=0.661; white blood cells,
P=0.772; platelets,
P=0.703) (
Table 1). Karyotype analysis of BM aspirates at diagnosis was available for 32 of the 51 patients. Ten of these 32 patients (31.3%) showed cytogenetic abnormalities, and among these 10 patients, only 4 showed hyperploidy whereas the remaining 6 showed a complex karyotype. No correlation was noted between karyotype abnormalities and differentiation status of neuroblasts in BM. Chemotherapy regimens and proportions of patients who underwent BMT during treatment were not significantly different among the patient groups (
Table 2).
2. Comparison of clinical and laboratory characteristics and prognosis according to differentiation status of neuroblasts in BM at diagnosis
Of the 13 patients who exhibited differentiating neuroblasts in BM at diagnosis, 7 were males and 6 were females, with age ranging from 0.8 to 9.5 yr (median, 3.6 yr). Among these patients, only 2 were younger than 2 yr at diagnosis. Of the 38 patients who did not exhibit differentiating neuroblasts in BM at diagnosis, 20 were males and 18 were females, with age ranging from 0.1 to 23.0 yr (median, 3.3 yr). Among these patients, 11 were younger than 2 yr. Clinical variables did not differ according to the differentiation status of neuroblasts in BM at diagnosis; these variables included gender (P=0.940), age (P=0.239), VMA (P=0.964), HVA (P=0.251), NSE (P=0.527), ferritin (P=0.495), LD (P=0.081), and blood cell counts (hemoglobin, P=0.341; white blood cells, P=0.559; platelets, P=0.863).
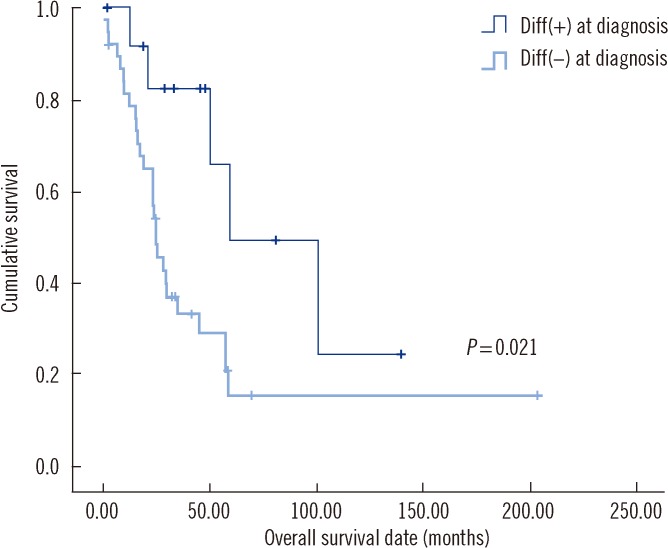
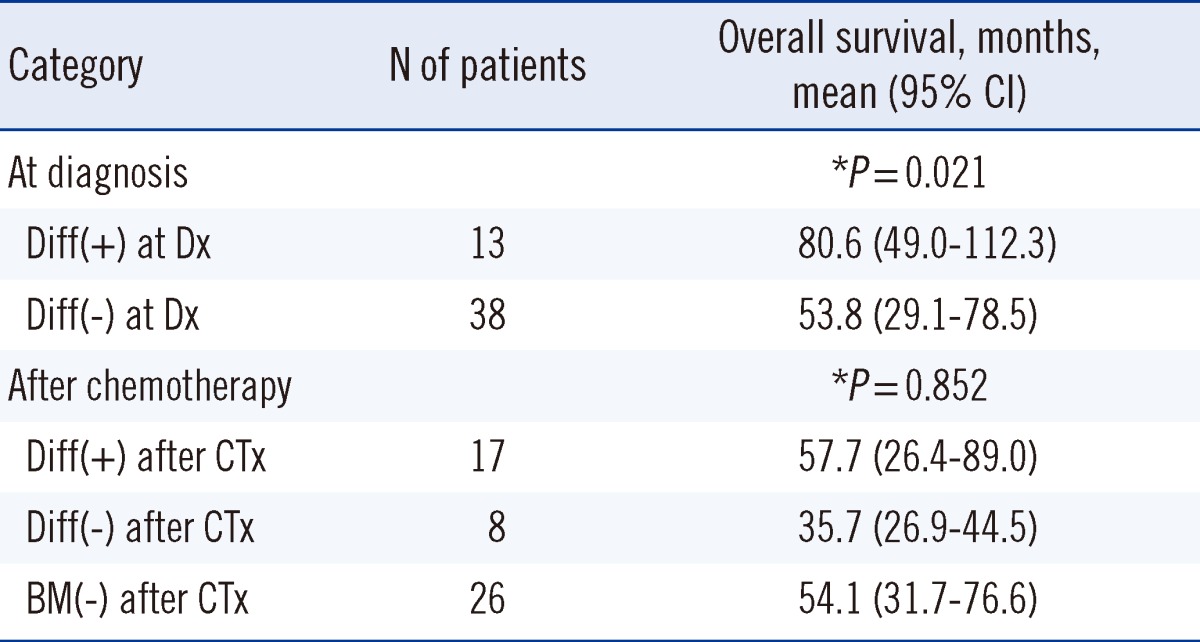
The presence of differentiating neuroblasts in BM at diagnosis was significantly associated with longer OS (80.6 months vs. 53.8 months,
P=0.021) (
Table 3,
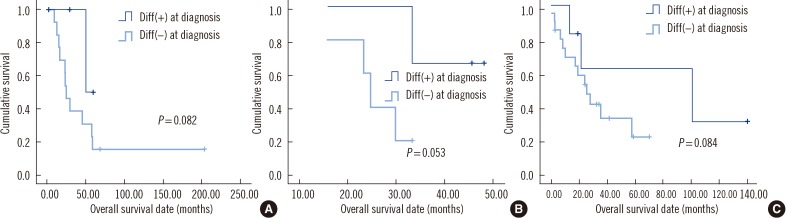
Fig. 2). Further analysis indicated that this was the case regardless of the differentiation status in BM after chemotherapy (
P=0.082 for a comparison between groups I-1 and II-1, members of which exhibited differentiating neuroblasts in BM after chemotherapy;
P=0.053 for a comparison between groups I-2 and II-2, members of which did not exhibit differentiating neuroblasts in BM after chemotherapy;
P=0.084 for a comparison between groups I-3 and II-3, members of which exhibited no BM metastasis after chemotherapy) (
Fig. 3).
3. Comparison of clinical and laboratory characteristics and prognosis with regard to differentiation status in BM after chemotherapy
Of the 17 patients who exhibited differentiating neuroblasts in BM after chemotherapy, 9 were males and 8 were females, with age ranging from 0.1 to 9.5 yr (median, 2.9 yr). Of the 8 patients who did not exhibit differentiating neuroblasts in BM after chemotherapy, 3 were males and 5 were females, with age ranging from 3.2 to 10.2 yr (median, 4.5 yr). Of the 26 patients with no BM metastasis after chemotherapy, 15 were males and 11 were females, with age ranging from 0.1 to 23.1 yr (median, 3.3 yr).
Clinical and laboratory variables did not differ among these 3 groups; these variables included gender (
P=0.606), age (
P=0.104), VMA (
P=0.420), HVA (
P=0.912), NSE (
P=0.663), ferritin (
P=0.433), LD (
P=0.521), and blood cell counts (hemoglobin,
P=0.698; white blood cells,
P=0.618; platelets,
P=0.537). Additionally, OS was similar among the 3 groups (patients with differentiating neuroblasts in BM after chemotherapy, 57.7 months; patients without differentiating neuroblasts in BM after chemotherapy, 35.7 months; patients with no BM metastasis after chemotherapy, 54.1 months;
P=0.852) (
Table 3). Further analysis indicated that the presence of differentiating neuroblasts in BM after chemotherapy was not significantly associated with OS regardless of the differentiation status of neuroblasts in BM at diagnosis (
P=0.545 in group I, members of which exhibited differentiating neuroblasts in BM at diagnosis;
P=0.944 in group II, members of which did not exhibit differentiating neuroblasts in BM at diagnosis).
4. Multivariate analysis of overall survival with regard to the differentiation status at diagnosis and after chemotherapy
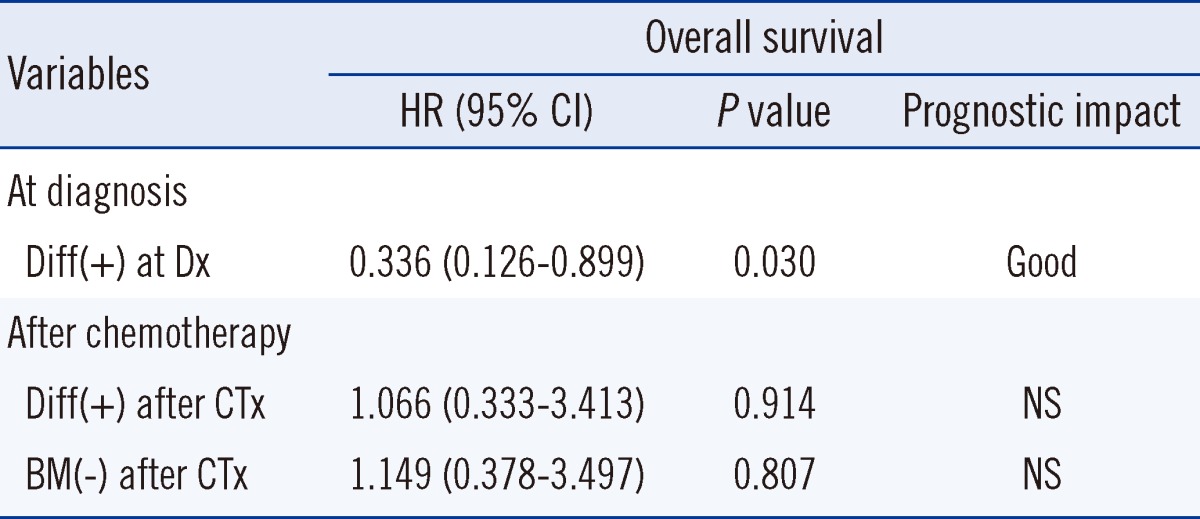
We subsequently performed a multivariate analysis, which demonstrated that differentiation of neuroblasts in BM at diagnosis was associated with a statistically significant positive prognostic impact on OS (hazard ratio, 0.336;
P=0.030). However, the presence of differentiation in BM after chemotherapy did not have any prognostic impact on OS (hazard ratio, 1.066;
P=0.914). Even in patients without BM involvement after chemotherapy, the prognostic impact was not evident (hazard ratio, 1.149;
P=0.807) (
Table 4).
DISCUSSION
NB maturation is the process by which a morphologically undifferentiated tumor becomes a mature ganglioneuroma [
1]. The INPC uses both cytoplasmic and nuclear enlargement as morphologic indicators of neuroblastic differentiation [
14]. Increasing numbers of differentiating neuroblasts are considered a sign of tumor maturation, suggesting a good prognosis [
14]. Several studies have reported that spontaneous maturation of primary tumors in patients who did not receive antitumor therapy is associated with improved survival rate and prognosis [
6-
8]. According to these reports, the factors that correlated with spontaneous maturation were age (<1 yr) and disease stage (stage 1, 2, and 4 tumors) [
15].
However, few reports have described the presence of differentiating neuroblasts after chemotherapy in the BM of patients with stage 4 NB. One report described the presence of differentiating neuroblasts in tissue from a patient with chemoresistant stage 4 NB after a 2-week treatment with [
131I] metaiodobenzylguanidine [
16]; however, this patient died 2 months later, suggesting that histological maturation after chemotherapy did not affect survival. Another study reported that 10 patients treated with conventional cytotoxic chemotherapy (with or without maturation-inducing agents) exhibited differentiating neuroblasts at advanced stages (3 or 4); however, this phenomenon did not correlate with prognosis [
15].
In this study, we found that 13 patients (25.5%) had differentiating neuroblasts in BM at diagnosis and 13 patients had differentiating neuroblasts in BM after chemotherapy. A variety of agents can induce neurite outgrowth in human NB cell lines, including retinoic acid, cyclic AMP-elevating agents, prostaglandin E1, papaverine, and nerve growth factor [
15,
17]; however, our patients did not receive these agents, and the chemotherapy regimen did not differ among the 6 patient groups. Consistent with previous studies, our results reveal that the presence of differentiating neuroblasts in BM at diagnosis and after chemotherapy is relatively common among patients with BM metastatic NB regardless of the use of differentiation-inducing agents. To our knowledge, our study is the first report that describes the presence of differentiating neuroblasts in BM at diagnosis and after chemotherapy in patients with BM metastatic NB.
In addition, we found that the presence of differentiating neuroblasts in BM at diagnosis was associated with significantly better prognosis even though other prognostic markers such as urinary VMA and HVA as well as serum NSE and LD levels did not differ among the groups categorized by differentiation status of neuroblasts in BM. Among all patient subgroups categorized by differentiation status of neuroblasts in BM after chemotherapy, the prognosis appeared to be better for patients who exhibited differentiating neuroblasts in BM at diagnosis. Our results are consistent with previous studies reporting that spontaneous maturation of primary tumors in patients who do not undergo antitumor therapy predicts better prognosis and longer survival. In contrast, the presence of differentiating neuroblasts in BM after chemotherapy was not associated with prognosis or OS, regardless of the differentiation status of neuroblasts in BM at diagnosis. These findings suggest that the presence of differentiating neuroblasts in BM after chemotherapy may indicate localized progression only.
During ganglion differentiation, Schwann cells are recruited from surrounding non-neoplastic tissue by neoplastic neuroblasts that produce mitogens and chemotactic factors. Schwann cells already present in the tumor tissue are thought to produce anti-proliferative and differentiation-inducing factors crucial to neuronal differentiation [
1]. Although Schwann cells are present in BM, they are difficult to identify in BM aspirates. Moreover, they cannot easily be observed on microscopy because myelin and axons cannot be stained using the May-Grunwald-Giemsa method, and only thick nerve bundles are stained using hematoxylin and eosin [
18]. Although we did not detect Schwann cells in BM aspirate specimens, the nerve bundles observed in BM biopsies indicated the presence of differentiating neuroblasts.
Generally, more highly differentiated tumors secrete more mature form of catecholamine [
19,
20]. Levels of HVA, which is a metabolite of dopamine, are often elevated in patients with mature NB. In contrast, VMA, which is a metabolite of epinephrine and norepinephrine, suggests the presence of a less differentiated tumor [
11]. Thus, patients with more mature NB are expected to have higher levels of HVA than VMA. However, in our study, VMA and HVA levels did not differ among the patient groups regardless of neuroblast differentiation status in BM at diagnosis or after chemotherapy.
Because NB does not appear in a homogeneous manner in BM, the results of an metaiodobenzylguanidine (MIBG) scan and BM biopsy may show discrepant findings, i.e., BM may be MIBG positive with no tumor cells observable in a BM biopsy. This discrepancy could bias the results of our study. Therefore, we evaluated the validity of an MIBG scan during the follow-up period of all 51 patients under investigation. Except for 9 patients who were initially diagnosed prior to 1995, 42 of the 51 (82.4%) patients underwent an MIBG scan during follow-up. The results of these MIBG scans corresponded perfectly with those of BM biopsies. The 17 patients who showed BM metastasis in BM biopsies at a particular time during the follow-up period were all MIBG positive at that time. The 25 patients who did not show BM metastasis in BM biopsies at a specific follow-up time were all MIBG negative at that time. Therefore, the negative predictive value of MIBG scans was 100%. The association between the differentiation status of neuroblasts at the primary site and in the BM metastatic lesion has not been evaluated adequately and therefore needs to be investigated. In our study, we often found concordance between the differentiation status of the primary tumor and the BM metastatic lesion at diagnosis. Indeed, 9 out of 13 cases with differentiated neuroblasts in BM at diagnosis also showed differentiating neuroblasts at the primary site. However, the prognostic impact of differentiation in the primary tumor was not evident from our analysis. A comprehensive study with more patients is required for accurate evaluation of the prognostic impact of differentiation in the primary tumor.
Our study has some limitations. First, the results of known prognostic markers (e.g., MYCN amplification, CD44 expression, Trk-A expression) could not be included in our analysis, and karyotype data were available only for two-thirds of patients. Therefore, a thorough multivariate analysis including these proven prognostic factors could not be performed. A more comprehensive study including these markers needs to be conducted in the future. Second, because we divided the 51 patients into 6 patient groups and then performed subgroup analysis, the numbers used for comparison were sometimes small, which could result in low statistical power. In the future, a study that includes larger numbers will be required to confirm the prognostic impact of the presence of differentiating neuroblasts in BM at diagnosis and after chemotherapy.
In conclusion, we found that the presence of differentiating neuroblasts in BM at diagnosis and after chemotherapy is common in patients with BM metastatic NB. The presence of differentiating neuroblasts in BM at diagnosis is associated with significantly better prognosis; however, the presence of differentiating neuroblasts in BM after chemotherapy is irrelevant to prognosis.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download