Abstract
Background
Procalcitonin (PCT), C-reactive protein (CRP), and white blood cells (WBCs) are inflammatory markers used to diagnose severe bacterial infections. We evaluated the diagnostic role of these markers and compared their accuracy for spontaneous bacterial peritonitis (SBP) associated with chronic severe hepatitis B (CSHB).
Methods
PCT and CRP concentrations, WBC count, and other hematological parameters were measured in serum from 84 well-characterized patients with CSHB, of whom 42 had SBP. Receiver operating characteristics (ROC) curve analysis was performed to assess the diagnostic accuracy.
Results
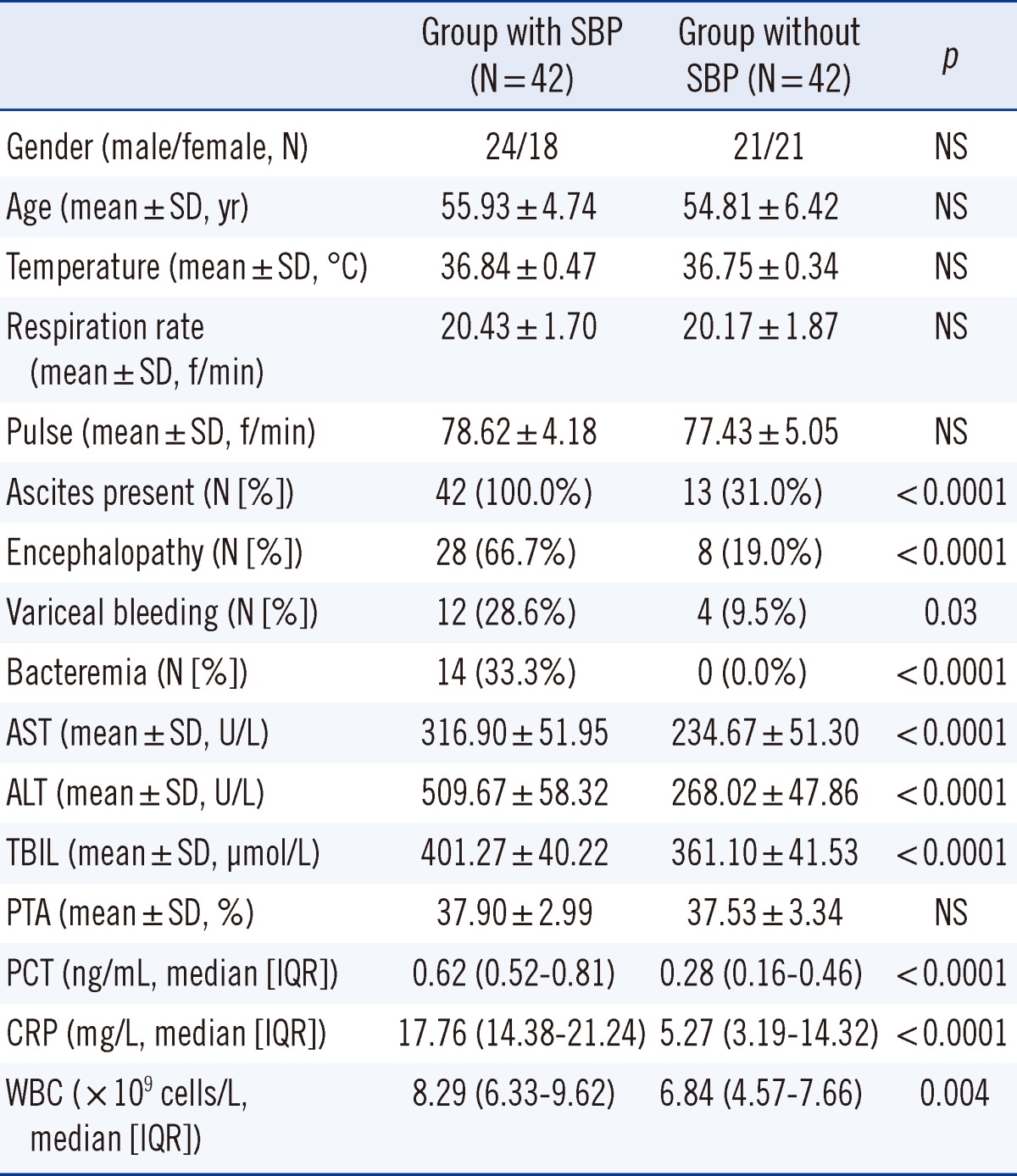
PCT and CRP concentrations were significantly higher in the CSHB patients with SBP (n=42) than CSHB patients without SBP (n=42). PCT and CRP concentrations were more accurate than WBC count for the diagnosis of CSHB-associated SBP. The optimal cutoff value of PCT was 0.48 ng/mL. The PCT concentration was significantly correlated with the CRP concentration and WBC count.
Hepatitis B virus (HBV) infection remains a global health problem, with more than 350 million chronically infected people; every year approximately 1 million people die from HBV-related diseases [1]. Notably, bacterial infections are a common cause of morbidity in patients with chronic severe hepatitis B (CSHB). Widespread bacterial infections can decompensate hepatic status and lead to death in patients with CSHB [2, 3]. Various infections lead to death in 30-50% patients with liver cirrhosis [2, 4]. Furthermore, bacterial infections are known to trigger complications of CSHB, including spontaneous bacterial peritonitis (SBP), variceal bleeding, hepatic encephalopathy, renal failure, and impairment in clotting factors [5].
A new inflammatory marker that could predict infections and assist physicians in making treatment decisions would be extremely useful. Inflammatory markers, such as C-reactive protein (CRP) and procalcitonin (PCT) [6] and white blood cells (WBCs) could be easily used for diagnosis and follow-up of several morbidities [6, 7]. CRP is synthesized mainly in the liver. Serum CRP concentrations were reported to be associated with metabolic syndrome and diabetes [8]. CRP has also been suggested as a predictive marker of cardiovascular events in patients with metabolic syndrome [8, 9] and may be correlated with the degree of tissue damage and the activity of the basal malignant disease [10-13]. A 116-amino acid prohormone of calcitonin, PCT is normally synthesized in the C cells of the thyroid gland. Other sources of PCT include liver and inflammatory cells. Thyroid-excised subjects continue to have PCT response during acute inflammation; therefore, the main site of PCT synthesis and its function are not yet completely clear [14-19]. The serum PCT concentrations increase in patients with bacterial infections or sepsis [20]. Similarly, altered levels of serum PCT have been well documented in chronic liver diseases and cirrhosis. However, serum PCT is not elevated in viral or autoimmune diseases of the liver [21].
Although some authors have used PCT as an inflammatory marker in the diagnosis of SBP [22], serum PCT has not been evaluated in the diagnosis of SBP in chronic hepatitis B patients. Therefore, we aimed to study the diagnostic role of serum PCT in CSHB patients with SBP and compared its diagnostic efficacy with those of CRP and WBCs.
Between June 2010 and June 2012, 84 well-characterized, consecutive patients with CSHB (male/female: 45/39, age: 48.31±2.74 yr) from the Infection Department of Renmin Hospital (University of Wuhan) were considered. Of these, 42 CSHB patients with SBP without any infection in other organs or sites were enrolled as the study group. The control group was defined as 42 CSHB patients without SBP, and these patients did not have any overt infection. The clinical and laboratory characteristics of the patients are presented in Table 1.
Chronic hepatitis B viral carriers was diagnosed based on (a) positive tests for hepatitis B surface antigen (HBsAg) for at least 6 months before hospitalization or (b) positive tests for HBsAg, anti-hepatitis B core antibody (HBcAb) at a high titer and negative tests or a low titer of IgM anti-hepatitis B core antibody (IgM-HBc) in patients with follow-up periods of >6 months before admission. Blood samples and clinical data, including the presence of ascites, encephalopathy, bacteremia, recent episode of variceal bleeding, temperature, respiration rate, pulse, and blood pressure, were taken at enrollment. The diagnosis of SBP was established by assessment of clinical symptoms, appearance of fever, and laboratory reports (polymorphonuclear cell count in ascites >0.25×109/L, a positive culture for any organism, or absence of an intraabdominal infection). Bacteremia was considered present, if the blood culture was positive for at least one organism when a documented primary infection was absent; blood cultures were performed for all patients. Furthermore, a contaminant was defined as a nonpathogenic microorganism [23], and cases of contamination were excluded from the analysis of bacteremia.
Clinical data were obtained retrospectively by thorough review of the patients' medical charts and included age, presence of ascites, encephalopathy, recent episode of variceal bleeding, bacteremia, AST, ALT, total bilirubin, prothrombin activity (PTA), etc.
At the time of admission, venous blood was drawn from all the patients and centrifuged at 1,000×g for 10 min at 4℃ after a full blood cell count. All samples were tested for PCT, CRT, and WBCs within 30 min after acceptance by the clinical laboratory. PCT quantification was performed using automated immunoanalysis with the Liaison analyzer (Diasorin, Saluggia, Italy). For CRP, an immunoturbidimetric assay with ADVIA Chemistry CRP_2R (Siemens Medical Solutions Diagnostics, Tarrytown, NY, USA) was used. The WBC count was determined using DxH 800 (Beckman Coulter, Pasadena, CA, USA). The limits of detection of these techniques were 0.04 ng/mL, 4 mg/L, and 0.5×109 cells/L for PCT, CRT, and WBC count, respectively.
Continuous variables were reported as mean (SD) or as median (interquartile range [IQR]), according to their homogeneity. Categorical variables were compared with the χ2-test, and continuous variables were compared with Student's t-test for parametric data or a Mann-Whitney test for medians of nonparametric data. To test the accuracies and cut-off values for different inflammatory markers, ROC curves were generated. The Spearman's correlation coefficient (rs) between PCT and other inflammatory markers was calculated for each group. The optimal cutoff value was determined by calculating the point on the ROC curve with the maximum Youden index (sensitivity-[1-specificity]) and the point with the shortest distance from the point (0,1) [(1-sensitivity)2+(1-specificity)2] for each inflammatory marker. Subsequently, the sensitivity, specificity, likelihood ratio of a positive (LR+) result, likelihood ratio of a negative (LR-) result, and diagnostic odds ratio were calculated for the different inflammatory markers. The optimal cutoff values of the different inflammatory markers were investigated for the diagnosis of CSHB patients with SBP. A 2-sided probability value of <0.05 was considered statistically significant. Data were analyzed using GraphPadPrism 5 (San Diego, CA, USA) and SPSS 17.0 (SPSS Inc, Chicago, IL, USA).
A total of 42 consecutive CSHB patients had clinically proven SBP, whereas the other 42 had no SBP at the time of polymorphonuclear cells counting (Table 1). There were no significant differences in gender, age, temperature, respiration, pulse, blood pressure, or PTA between the 2 groups. However, patients with SBP had specific complications (such as ascites, encephalopathy, variceal bleeding, and bacteremia) more frequently than patients without SBP. ALT, AST, and total bilirubin, were significantly higher in the SBP group than in the non-SBP group.
A significant positive correlation was observed between PCT and CRP concentrations (P<0.0001, rs=0.69), and a less strong, but still significant, correlation was observed between PCT concentration and WBC count (P=0.001, rs=0.36).
As determined by ROC curve analysis (Fig. 2), the accuracies of PCT concentration (area under the curve [AUC], 0.89; 95% (confidence interval) [CI], 0.81-0.96) and CRP concentration (AUC, 0.86; 95%CI, 0.78-0.94) for identifying CSHB patients with SBP were significantly higher (P<0.01) than those for WBC count (AUC, 0.68; 95% CI, 0.57-0.78). The AUC-ROC for the PCT concentration was not significantly different from that for the CRP concentration.
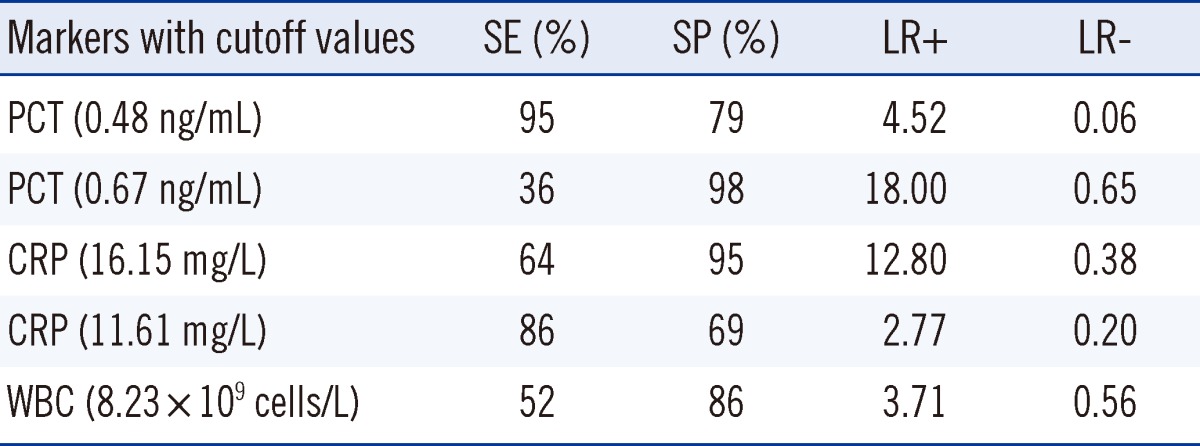
The optimal cutoff value for PCTwas 0.48 ng/mL. Meanwhile, the sensitivity and specificity of PCT for CSHB patients with SBP were 95% and 79%, respectively, at the cutoff of 0.48 ng/mL. The optimal diagnostic cutoff value of CRP was 16.15 mg/L. The sensitivity and specificity of CRP in CSHB patients with SBP were 64% and 95%, respectively, at the cutoff of 16.15 mg/L. The optimal cutoff value for WBCs was 8.23×109 cells/L.The sensitivity and specificity of WBCs for CSHB patients with SBP were 52% and 86%, respectively, at the cutoff of 8.23×109 cells/L. Table 2 shows a comparison of the parameters of serum PCT, CRP, and WBC count for the diagnostic accuracy of CSHB patients with SBP at different cutoff values. The PCT had a maximum LR+ for the diagnosis of CSHB patients with SBP at the cutoff of 0.48 ng/mL. The LR+ of PCT for CSHB patients with SBP was higher than that of CRP at a cutoff of 16.15 mg/L and WBC count at the cutoff of 8.23×109 cells/L.
CSHB is a serious chronic liver disease that eventually results in acute decompensation of liver function. Usually, one or more reversible precipitating events such as superimposed acute hepatic necrosis, SBP, worsening renal function, or gastrointestinal bleeding lead to the acute decompensation of liver function. Chronic severe hepatitis often involves acute-on-chronic liver failure and chronic liver failure [24]. In China, most fulminant hepatitis patients have chronic liver failure caused by hepatitis virus B. Patients with CSHB are immunocompromised and thus are highly susceptible to the dissemination of infections such as SBP that worsen hepatic function and results in severe disease complications [25, 26]. Therefore, early diagnosis and active treatment of infections are vital. Obviously, it is crucial to determine a new clinical laboratory test for predicting infections and assist the physician in making treatment decisions. In the present study, we assessed the clinical utility of different inflammatory markers (PCT, CRP, and WBCs) for identifying bacterial infections in CSHB.
In our study, gender, age, temperature, respiration rate, pulse, blood pressure, and PTA were similar between the 2 groups. The disease-specific complications (ascites, encephalopathy, variceal bleeding, and bacteremia), including ALT, AST, and total bilirubin were strikingly different between groups (P<0.05).
The concentrations of PCT and CRP and the WBC count were significantly higher in the CSHB patients with SBP than in those without SBP. Furthermore, there were significant correlations between PCT and other inflammatory markers such as the WBC count and CRP. However, PCT and CRP concentrations were found to be better than WBC count for predicting CSHB with SBP, and the accuracy of the PCT concentration was not significantly different from that for the CRP concentration.
The diagnostic and prognostic value of serum PCT level in liver diseases has been evaluated in several studies, with relatively consistent results [27-32]. In patients with decompensated liver cirrhosis, a high PCT concentration showed a high sensitivity and specificity for bacterial infections [33]. In these studies, the serum PCT was significantly elevated in CSHB patients with SBP compared with those without SBP, but the median values varied: 0.74 ng/mL [28], 2.8 ng/mL [29], 3.2 ng/mL [7], 9.8 ng/mL [11], and 10.1 ng/mL [12]. We also found significantly elevated PCT concentrations in our CSHB patients with SBP, but the median value was low: 0.62 ng/mL. Furthermore, in this study, PCT concentration at the optimal cutoff of 0.48 ng/mL had the best sensitivity (95%), specificity (79%), and AUC-ROC (0.89) for SBP in CSHB patients. In the present cohort, the PCT concentration increased significantly in CSHB patients with SBP compared to those without SBP. These discrepancies can be due to the presence of bacterial infections; on the other hand, patients with acute viral hepatitis in with a CSHB background without proven bacterial infections show a high serum PCT with disease progression [28]. Therefore, we should measure serum PCT when the severity of disease is similar between groups.
CRP and WBC have been used as inflammatory markers of bacterial infections, even in liver diseases [34]. However, many questions on the optimal application of CRP and WBC in this patient population have not been addressed. It is vital to assess the determination power of CRP and WBC. In previous clinical studies involving 20 to 127 patients, CRP and WBC proved to be effective markers of bacterial infections in patients with liver diseases, but they had diverse diagnostic accuracies and cutoff values [27-29, 35-37]. A possible explanation for this variance may be the differences in the type of liver disease in between patients. In the present study, CRP at the optimal cutoff of 16.15 mg/L had the best sensitivity (64%), specificity (95%), and AUC-ROC (0.86). The optimal cutoff value of WBC count was 8.23×109 cells/L, with a lower sensitivity (52%), specificity (86%), and AUC-ROC (0.68). This suggests that the WBC count is inferior to the serum PCT for the diagnosis of CSHB patients with SBP.
In conclusion, serum PCT and CRP concentrations were more accurate than WBC count for the diagnosis of CSHB-associated SBP. However, this study only included a small cohort (n=42) of patients with SBP. Larger samples and more homogeneous groups of CSHB patients with SBP should be used for further studies in order to confirm our results.
Acknowledgements
This work was supported by a grant from the National Clinical Key Specialty Construction Projects to the Department of Clinical Laboratory of Renmin Hospital of Wuhan University.
References
1. Lavanchy D. Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention. J Clin Virol. 2005; 34:S1–S3. PMID: 16461208.

2. Borzio M, Salerno F, Piantoni L, Cazzaniga M, Angeli P, Bissoli F, et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001; 33:41–48. PMID: 11303974.

3. Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008; 28:26–42. PMID: 18293275.

4. Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993; 18:353–358. PMID: 8228129.

5. Thalheimer U, Triantos CK, Samonakis DN, Patch D, Burroughs AK. Infection, coagulation, and variceal bleeding in cirrhosis. Gut. 2005; 54:556–563. PMID: 15753544.

6. Massaro KS, Costa SF, Leone C, Chamone DA. Procalcitonin (PCT) and C-reactive protein (CRP) as severe systemic infection markers in febrile neutropenic adults. BMC Infect Dis. 2007; 7:137. PMID: 18034890.

7. Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999; 19:972–978. PMID: 10195925.
8. Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000; 321:199–204. PMID: 10903648.

9. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000; 342:836–843. PMID: 10733371.

10. Heney D, Lewis IJ, Evans SW, Banks R, Bailey CC, Whicher JT. Interleukin-6 and its relationship to C-reactive protein and fever in children with febrile neutropenia. J Infect Dis. 1992; 165:886–890. PMID: 1569338.

11. Katz JA, Mustafa MM, Bash RO, Cash JV, Buchanan GR. Value of C-reactive protein determination in the initial diagnostic evaluation of the febrile, neutropenic child with cancer. Pediatr Infect Dis J. 1992; 11:708–712. PMID: 1448309.

12. Engel A, Mack E, Kern P, Kern WV. An analysis of interleukin-8, interleukin-6 and C-reactive protein serum concentrations to predict fever, gram-negative bacteremia and complicated infection in neutropenic cancer patients. Infection. 1998; 26:213–221. PMID: 9717678.

13. Lehrnbecher T, Venzon D, de Haas M, Chanock SJ, Kühl J. Assessment of measuring circulating levels of interleukin-6, interleukin-8, C-reactive protein, soluble Fc gamma receptor type III, and mannose-binding protein in febrile children with cancer and neutropenia. Clin Infect Dis. 1999; 29:414–419. PMID: 10476751.
14. Giamarellos-Bourboulis EJ, Grecka P, Poulakou G, Anargyrou K, Katsilambros N, Giamarellou H. Assessment of procalcitonin as a diagnostic marker of underlying infection in patients with febrile neutropenia. Clin Infect Dis. 2001; 32:1718–1725. PMID: 11360214.

15. al-Nawas B, Shah PM. Procalcitonin in patients with and without immunosuppression and sepsis. Infection. 1996; 24:434–436. PMID: 9007590.

16. Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993; 341:515–518. PMID: 8094770.

17. Gendrel D, Raymond J, Assicot M, Avenel S, Lefèvre H, Ravilly S, et al. Procalcitonin, C-reactive protein and interleukin 6 in bacterial and viral meningitis in children. Presse Med. 1998; 27:1135–1139. PMID: 9767794.
18. Whang KT, Steinwald PM, White JC, Nylen ES, Snider RH, Simon GL, et al. Serum calcitonin precursors in sepsis and systemic inflammation. J Clin Endocrinol Metab. 1998; 83:3296–3301. PMID: 9745444.

19. Giamarellou H, Giamarellos-Bourboulis EJ, Repoussis P, Galani L, Anagnostopoulos N, Grecka P, et al. Potential use of procalcitonin as a diagnostic criterion in febrile neutropenia: experience from a multicentre study. Clin Microbiol Infect. 2004; 10:628–633. PMID: 15214875.

20. Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004; 39:206–217. PMID: 15307030.

21. Maruna P, NedelníkováK , Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000; 49:S57–S61. PMID: 10984072.
22. Su DH, Zhuo C, Guan J, Liao K, Cheng WB, Cheng H, et al. Value of serum procalcitonin levels in predicting spontaneous bacterial peritonitis. Hepatogastroenterology. 2013; 60:641–646. PMID: 23159389.
23. Ramsook C, Childers K, Cron SG, Nirken M. Comparison of blood culture contamination rates in a pediatric emergency room: newly inserted intravenous catheters versus venipuncture. Infect Control Hosp Epidemiol. 2000; 21:649–651. PMID: 11083181.
24. Liu Q, Liu Z, Wang T, Wang Q, Shi X, Dao W. Characteristics of acute and sub-acute liver failure in China: nomination, classification and interval. J Gastroenterol Hepatol. 2007; 22:2101–2106. PMID: 18031366.

25. Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008; 28:26–42. PMID: 18293275.

26. Thalheimer U, Triantos CK, Samonakis DN, Patch D, Burroughs AK. Infection, coagulation, and variceal bleeding in cirrhosis. Gut. 2005; 54:556–563. PMID: 15753544.

27. Bota DP, Van Nuffelen M, Zakariah AN, Vincent JL. Serum levels of C-reactive protein and procalcitonin in critically ill patients with cirrhosis of the liver. J Lab Clin Med. 2005; 146:347–351. PMID: 16310518.

28. Elefsiniotis IS, Skounakis M, Vezali E, Pantazis KD, Petrocheilou A, Pirounaki M, et al. Clinical significance of serum procalcitonin levels in patients with acute or chronic liver disease. Eur J Gastroenterol Hepatol. 2006; 18:525–530. PMID: 16607149.

29. Viallon A, Zeni F, Pouzet V, Lambert C, Quenet S, Aubert G, et al. Serum and ascitic procalcitonin levels in cirrhotic patients with spontaneous bacterial peritonitis: diagnostic value and relationship to pro-inflammatory cytokines. Intensive Care Med. 2000; 26:1082–1088. PMID: 11030164.

30. Spahr L, Morard I, Hadengue A, Vadas L, Pugin J. Procalcitonin is not an accurate marker of spontaneous bacterial peritonitis in patients with cirrhosis. Hepatogastroenterology. 2001; 48:502–505. PMID: 11379342.
31. Connert S, Stremmel W, Elsing C. Procalcitonin is a valid marker of infection in decompensated cirrhosis. Z Gastroenterol. 2003; 41:165–170. PMID: 12592597.
32. Papp M, Vitalis Z, Altorjay I, Tornai I, Udvardy M, Harsfalvi J, et al. Acute phase proteins in the diagnosis and prediction of cirrhosis associated bacterial infections. Liver Int. 2012; 32:603–611. PMID: 22145664.

33. Viallon A, Zeni F, Pouzet V, Lambert C, Quenet S, Aubert G, et al. Serum and ascitic procalcitonin levels in cirrhotic patients with spontaneous bacterial peritonitis: diagnostic value and relationship to pro-inflammatory cytokines. Intensive Care Med. 2000; 26:1082–1088. PMID: 11030164.

34. Runyon BA. Ascitic fluid and serum C-reactive protein concentrations in patients with and without peritonitis. Am J Clin Pathol. 1986; 86:773–775. PMID: 3788865.

35. Tsiakalos A, Karatzaferis A, Ziakas P, Hatzis G. Acute-phase proteins as indicators of bacterial infection in patients with cirrhosis. Liver Int. 2009; 29:1538–1542. PMID: 19659507.

36. Lin ZY, Chuang WL, Dai CY, Yu ML, Chen SC, Hsieh MY, et al. Clinical application of serum C-reactive protein measurement in the detection of bacterial infection in patients with liver cirrhosis. Kaohsiung J Med Sci. 2002; 18:121–126. PMID: 12149826.
37. Li CH, Yang RB, Pang JH, Chang SS, Lin CC, Chen CH, et al. Procalcitonin as a biomarker for bacterial infections in patients with liver cirrhosis in the emergency department. Acad Emerg Med. 2011; 18:121–126. PMID: 21276124.

Fig. 1
(A) Distribution of the serum concentrations of procalcitonin (PCT) and (B) C-reactive protein (CRP) and (C) white blood cell (WBC) count in chronic severe hepatitis B (CSHB) patients with or without spontaneous bacterial peritonitis (SBP).
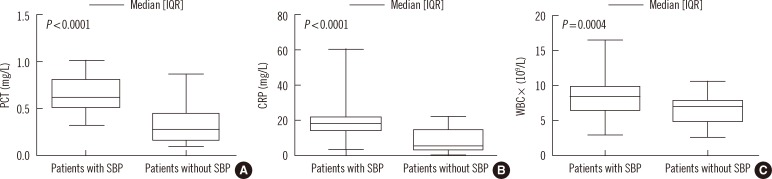
Fig. 2
ROC curve showing the diagnostic values of PCT, CRP, and WBC for the differentiation between CSHB patients with and without SBP. Areas under the curve were calculated for PCT (AUC, 0.89; 95% CI, 0.81-0.96; sensitivity 95% and specificity 79% at the optimal cutoff of 0.48 mg/L), CRP (AUC, 0.86; 95% CI, 0.78-0.94; 64%/95% at 16.15 mg/L), and WBC (AUC, 0.68; 95% CI, 0.57-0.78; 52%/86% at 8.23×109 cells/L).
Abbreviations: AUC, area under the curve; CRP, C-reactive protein; CSHB, chronic severe hepatitis B; PCT, procalcitonin; SBP, spontaneous bacterial peritonitis; WBC, white blood cell.

Table 1
Clinical and laboratory characteristics of chronic severe hepatitis B (CSHB) patients with or without spontaneous bacterial peritonitis (SBP)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download