Abstract
Background
Documentation is very important; a considerable number of documents exist for use in accreditation inspection. However, most laboratories do not effectively manage the processes of documentation, organization, and storage. The purpose of this study was to facilitate the establishment of a strategically effective and sustainably standardized document management system.
Methods
A document code formatting system was modified by comparing the document list data received from 3 major university hospitals. In addition, a questionnaire regarding document code standardization was created and sent to 268 institutes to establish document classifications and generate a standard coding scheme. A computerized document management system was developed.
Results
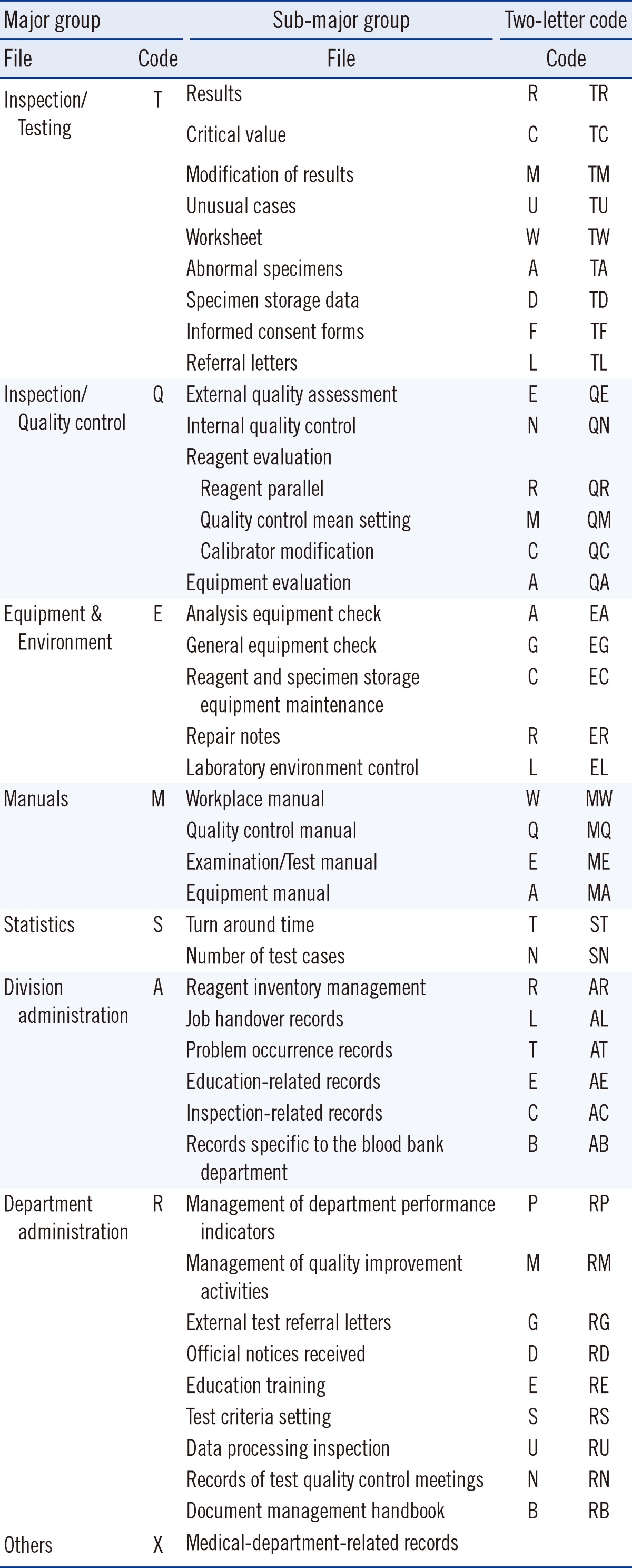
Only 32% (8 out of 25 institutes) answered that they were able to identify all of the document types and their numbers. In total, 76% of institutes (19 out of 25) answered that a systematic document management system was necessary. Disorganized document files were systemized by classifying them into 8 major groups according to their characteristics: patient test records (T), test quality control (Q), manuals (M), equipment and environment management (E), statistics (S), division administration (A), department administration (R), and others (X).
Laboratory medicine is the fastest growing among all specialized medical fields in terms of the development and introduction of new medical technologies. Recently, there have been growing needs to standardize medical laboratory test results in various areas of laboratory medicine and establish a standardized global system that is applicable across all fields of medical laboratory science.
In the United States, the College of American Pathologists (CAP) has implemented a laboratory standardization and accreditation system since 1962 and has continued to improve laboratories' quality through program development and improvement of its accreditation systems [1]. In Korea, accrediting evaluations and accreditations through the Korean Laboratory Accreditation Program (KLAP) were started in 1999 in order to improve the quality, accuracy, and reliability of laboratory work [2, 3]. At present, 268 laboratories participate in the KLAP. This allows for the systematic implementation of external and internal clinical laboratory quality assurance; quality control of safety, human resources, and equipment management; and laboratory standardization.
The KLAP inspection checklists were developed by considering domestic situations and referring to various foreign accreditation programs, including that of the CAP; they comprise 1,539 questions divided into 12 areas, including laboratory management [2, 3]. Each area consists of sub-items covering external quality assessment, quality improvement, internal quality control, laboratory test management by field, personnel, physical facilities, and laboratory safety. The checklist items regarding laboratory management include ones on specimen collection and handling, water quality control, glassware washing, assessment of laboratory skills, and laboratory computer services. Scores are given to these items, and revisions are made annually, including corrections and updates.
Documentation is very important in most cases, and a considerable number of documents exist for the purpose of accreditation inspection. However, most laboratories do not effectively manage the processes of documentation, organization, and storage; even within a single laboratory, document titles and formats vary among content areas. To improve this, a standard regulation that governs document management systems was recently added to the KLAP inspection checklist for laboratory management (01.201.380: "Is there a document management system?"). However, each hospital laboratory has only this item in its handbook and lacks concrete methods and detailed regulations for standardization; this only worsens existing within-laboratory confusion, which may create problems with inter-laboratory communication and information compatibility. Ineffective database management may even lead to financial losses; therefore, the systematic and practical management of document management systems through the process of document code standardization will greatly improve quality in the field of laboratory medicine.
The CAP has also expressed the need for document management as part of its Laboratory Accreditation Program. Although the CLSI guideline describes general principles of documentation management in CLSI document GP2-A5 [4], there are presently no detailed regulations or guidelines regarding document code standardization-not even in pertinent international guidelines. The laboratory general checklist of the 2010 CAP accreditation program has an item, GEN.20375 document control, which is intended to check whether a document control system exists.
The purpose of this study was to facilitate the establishment of a strategically effective and sustainably standardized document management system by developing methods for document classification and document code standardization in order to improve the quality of laboratory medicine in the medical laboratories of domestic hospitals.
The document code format was modified by comparing the document list data received from 3 tertiary hospitals (>1,000 beds).
A questionnaire (Fig. 1) regarding document code standardization was created and sent to 268 institutes; the responses received from 25 institutes were used to establish the document classifications and generate a standard coding scheme. The documents used in each department and unit within a laboratory were classified, and descriptive document titles were grouped together for common characteristics. Thus, document titles were standardized to facilitate effective document management in each department and unit. A document code format was developed from them.
On the bases of the document classification and standard code scheme generated above, a database and data input and output functions were established; moreover, a document management system was established to enable document scanning, storage, and searching.
We received responses from 25 institutes to which we sent the questionnaire. Regarding the number of laboratory tests performed, 2, 8, 4, 6, 2, and 2 institutes performed less than 1,000,000, 1,000,000-2,999,999, 3,000,000-4,999,999, 5,000,000-9,999,999, 10,000,000-20,000,000, and more than 20,000,000 laboratory tests, respectively. One institute did not respond to this question.
The representatives of only 32% (8 out of 25) of the institutes answered that they managed all of the document types and numbers. Meanwhile, 40% of the institutes had document system manuals, and 36% gave unique identification numbers to their documents. In total, 76% of the institutes (19 out of 25) responded that a systematic document management system was necessary, confirming its desirability in the majority of institutes. In addition, 95.2% admitted that their documents had different titles in different sections within the same laboratory and highlighted the need to standardize document titling.
A comparison between the records of tertiary hospitals (>1,000 beds) clarified that different names and classifications are given to the same types of documents. The titles and locations of specific identifiers within document names varied even within the same hospital (Table 1).
We defined the areas according to document type and characteristics based on the documents originating from the investigated tertiary hospitals, and systemized the document identification scheme by major and sub-major groups. A combination of alphabetical and numeric characters was used to standardize the document identification system. The major groups included patient test records (T), test quality control (Q), manuals (M), equipment and environment management (E), statistics (S), division administration (A), department administration (R), and others (X; Table 2). Patient test records which are labeled as "T" includes test-related troubleshooting, referral letters, informed consent forms, external test-related documents, test results, and work lists. This encompasses sub-major groups, such as records of patient outcomes, reports of critical values, results correction, unusual cases, worksheets, abnormal specimens, specimen storage, informed consent forms, and referral letters. Other files and documents are labeled "X," which includes discussion notes from internal consultations.
Documents were classified according to their characteristics. A 2-letter code was assigned to each document type, as shown in Table 2; the first letter represents the major group, and the second letter represents the sub-major group.
The last step was to complete the document coding scheme by linking the following 4 parts: (1) an abbreviation for the concerned institute; (2) department and unit, using the abbreviations for test units, including automated laboratory, diagnostic blood test unit, and microbiology unit; (3) document characteristics (divided into 7 categories in the case of the National Cancer Center), assigned as 2-digit serial numbers for each test or analyzer; and (4) the issuance frequency, in terms of yearly, monthly, quarterly, or semiannual frequency. Thus, unique document identification codes were generated by linking these 4 parts with hyphens. Documents completed quarterly, semiannually, and annually instead of monthly were coded as Q1, H1, and Y1, respectively. The following is an example of a document identification number representing a reagent evaluation performed in March 2011 in the automated laboratory:
NCC represents the National Cancer Center; LM represents the Department of Laboratory Medicine; AA represents clinical chemistry; QR01 represents quality control, parallel reagents, and the serial number; and 1103 represents March 2011 (i.e., the first 2 numbers designate the year, and the last 2 designate the month).
Once files were given identification codes, the problem of non-standardized file titles was addressed by first assigning sub-major codes according to document characteristics and subsequently adding detailed descriptions of records (e.g., "Internal Quality Control-Proficiency test"). This ordering was designed to ensure consistency and standardization.
The documents were scanned according to month. Algorithms were created to sort and store the documents automatically by extracting standardized document codes, and the scanned documents were formatted to enable storage as PDF files using an image converter (Fig. 2). To facilitate easy review of the documents, a document viewer was formatted to enable ListView (arranged by file name), TreeView (arranged by grouping system), monthly view, and searching (Fig. 3).
The results of the questionnaire survey revealed that most institutes consider document standardization to be very important. Furthermore, these results confirmed the importance of interdepartmental and inter-hospital standardization of document titles, justifying the objective of the present study. The inspection checklist currently applied to the KLAP accreditation inspection comprises over 1,500 items spanning 12 areas. Documentation is essential for the inspection. To date, hospital laboratories have created various types of documents according to their needs without abiding by any set regulations. Prior to standardization, we gathered laboratory directors' opinions on the necessity of a document control system and standardization thereof. The results confirmed the urgent need for a systematic and standardized document management system. Among the institutes answering that document management was practiced, only 32% of interviewees (8 out of 25) answered that they could identify the document types and their numbers. This means that the laboratory documents prepared for accreditation inspections were not managed properly in most institutes. However, 40% of the institutes had document system manuals and gave their documents unique identification numbers. In addition, 95.2% recognized that the same types of documents had different titles in different sections within the same laboratory and highlighted the necessity of standardization of document titling.
The GP-A5 guideline of CLSI presents the important components of procedure writing and management for clinical laboratories, especially those focused on the development of laboratory procedures [4]. Our study focused on standardization of document identification, and we presented the implementation of a document management system with our scheme. We propose a method of systemizing disorganized document files by classifying them according to their characteristics into 8 major groups: files and documents belonging to each major group were then organized into sub-major groups and given appropriate codes. In addition to this coding scheme, file and document titling were standardized through unification of the designations used by different departments. This enables systematic document management and provides a basic framework for compatibility in document exchange among hospitals.
Towards the beginning of the study, serial numbers of 2 or more digits were assigned to the documents; however, this practice was discontinued, because many documents did not use serial numbers. Furthermore, version-based management was not addressed because of its lack of practical relevance. The letters "I" and "O" were excluded from the coding scheme to prevent confusion with "one" and "zero," respectively, and any resulting computerization errors. Reagent evaluation had too many items to differentiate by document codes alone. Such criteria and classifications will frequently be useful in the future generation and organization of various documents. If hospital laboratories nationwide adopt such a systematic and effective system of document code standardization, the efficacy and convenience of communication within and among laboratories will be enhanced; thus, quality improvement in the field of laboratory medicine will be facilitated.
Our standardized documents can be managed electronically. Our institution scans documents and files after 2 yr storage; the scanned items are automatically converted into PDF files and stored automatically according to their assigned codes. The electronic system also enables review and retrieval by keyword, time period, and index.
Herein, we provide a basic framework to facilitate the systematic classification and management of various documents that serve as evidence in initiatives taken by each laboratory to assure accurate test results and quality improvement, which are the main inspection targets for accreditation. Besides its utility for onsite inspection, such standardization of document classification and coding will also enable systematic management and review and contribute to effective quality control in laboratories. Our document management system made periodic review of documents possible with reasonable effort.
The document management system should include processes of document identification, timely review and approval of both new and formerly approved documents, and retention and discarding of documents [4]. Especially, management of timely review and approval of formerly approved documents is very important.
One limitation of this study was that our results were not compliant with the International Organization for Standardization (ISO); further, the terms described in this study did not fully match CLSI terms, because the identification codes originated from document types in Korean laboratories whose situations do not seem identical with those of laboratories in the United States. Our study was not implemented in other hospitals. Thus, further efforts are necessary to generalize our document identification scheme.
Acknowledgements
This work was supported by the 2011 Quality Improvement Fund of the Laboratory Medicine Foundation.
References
1. Wagner LR. The College of American Pathologists, 1946-1996: laboratory standards. Arch Pathol Lab Med. 1997; 121:536–541. PMID: 9167614.
2. Lee WG, Kwak YS, Lee DH, Hwang YS, Lee KN. Clinical pathology laboratory inspection and accreditation in Korea I: development of the system and its trial. Korean J Clin Pathol. 2001; 21:86–92.
3. Lee WG, Kwak YS, Lee DH, Hwang YS, Lee KN. Laboratory inspection and accreditation in Korea II: analysis of the first round inspection. Korean J Lab Med. 2003; 23:363–369.
4. Clinical and Laboratory Standards Institute. Laboratory documents: developement and control; Approved guideline. GP2-A5. 5th ed. Wayne, PA: Clinical and Laboratory Standards Institute;2006.
Fig. 2
Schematic flow diagram of the Laboratory Medicine Document Management System (LMDMS). The document identification code in the header (in the present example, NCC-LMDM-EC01-1111) is extracted from the scanned file (WordPerfect file, WPF) (1). The image file is converted into a PDF file, which is stored on the document server (3), and the extracted document identification code and other document information (e.g., file tracking information) is stored on the database server (DB server) (2, 4).
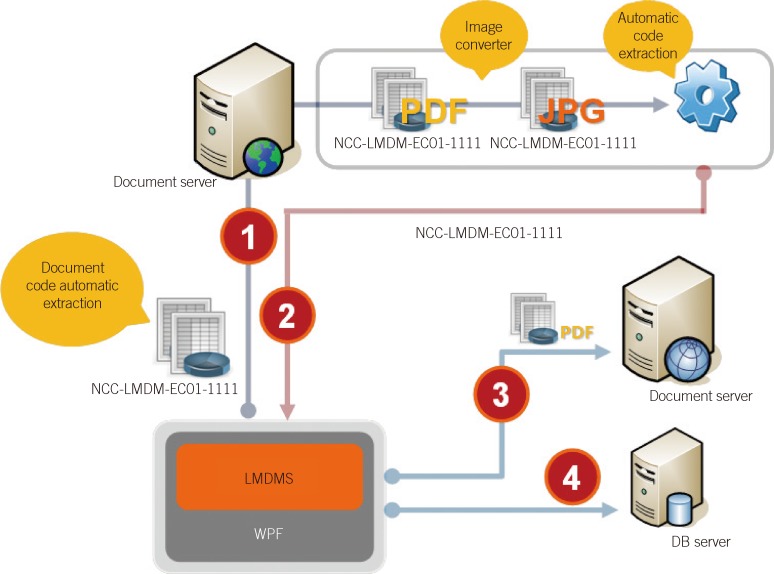
Fig. 3
Screenshot of the document display system. Three different searching tabs are provided as ListView tab (file-name-oriented), TreeView tab (classification-oriented), and Document search tab, which is accessible for monthly query and search, and easy for browsing documents. The document (NCC-LMDM-EC01-1112) for daily temperature check of a refregerator of the diagnostic immunology division in our laboratory can be seen in the TreeView tab.
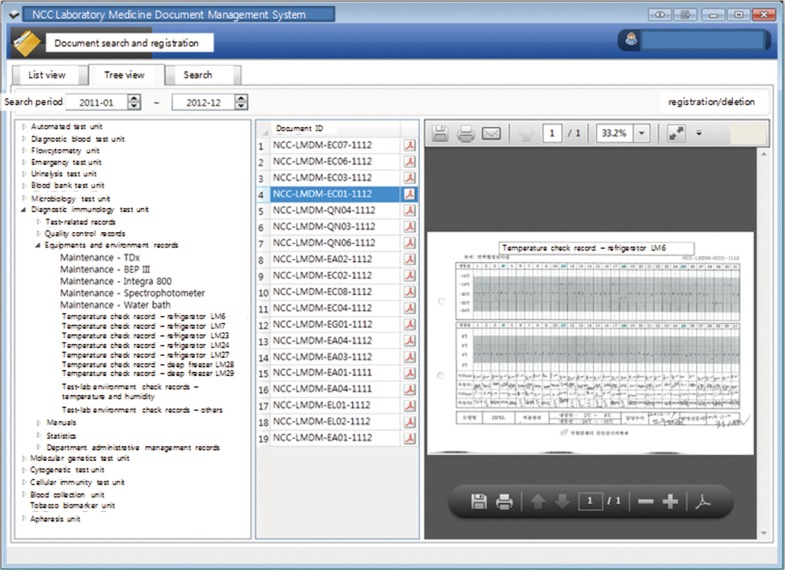
Table 1
Examples of the document items of 3 tertiary hospitals (> 1,000 beds)
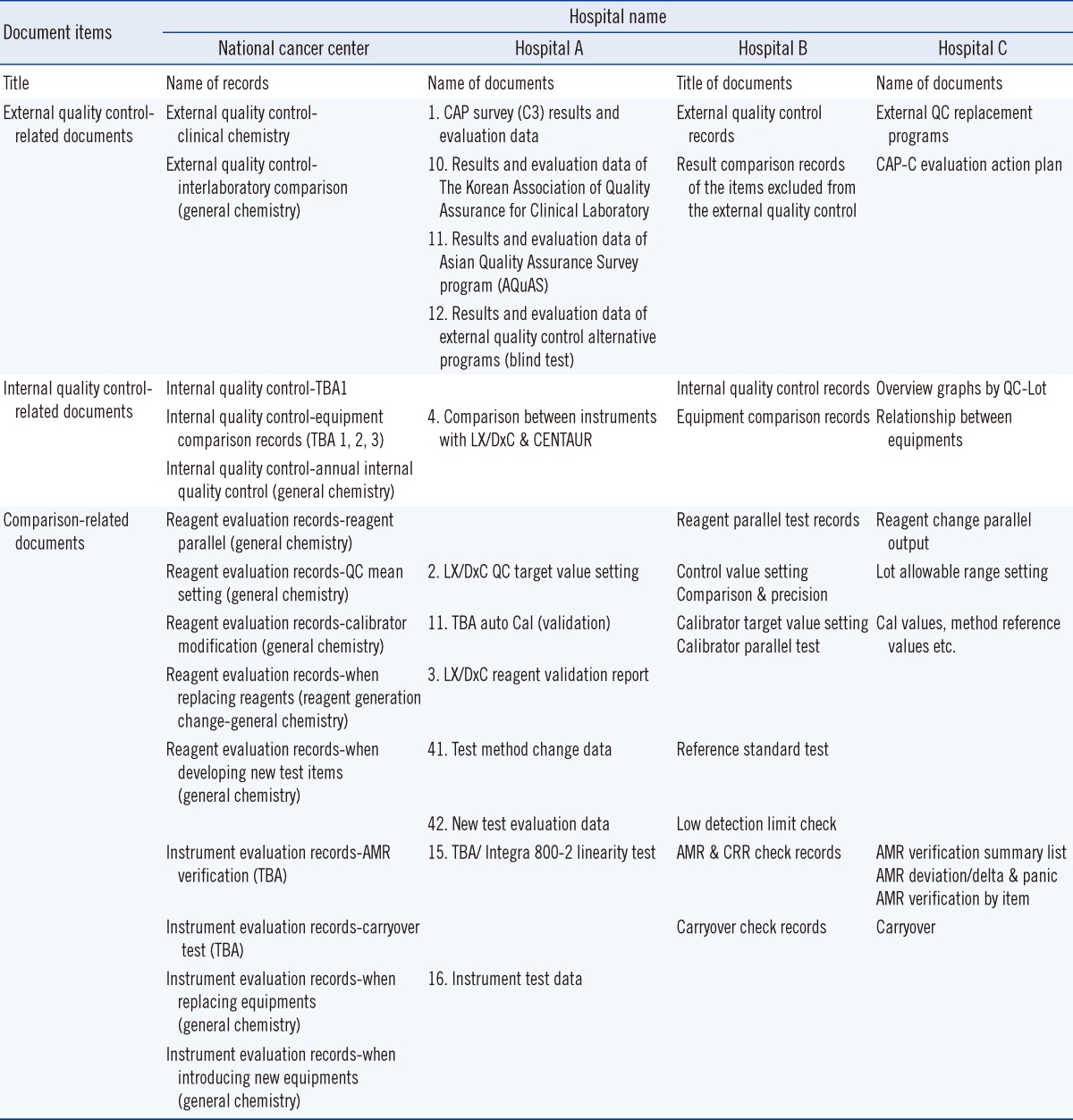
Abbreviations: CAP, College of American Pathologists; QC, quality control; TBA, Toshiba TBA-200FR NEO biochemical analyzer (Toshiba Medical Systems, Tokyo, Japan); LX/DxC, Beckman Coulter Synchron LX/DxC chemistry analyzer (Beckman Coulter, Brea, CA, USA); Cal, calibration; AMR, analytical measurement range; CRR, clinical reportable range.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download