Abstract
Background
Early diagnosis of HIV infection reduces morbidity and mortality. Fourth-generation HIV detection assays are more sensitive because they can detect p24 antigen as well as anti-HIV antibodies. In this study, we evaluated the performance of a new fourth-generation ADVIA Centaur HIV antigen/antibody combo (CHIV) assay (Siemens Healthcare Diagnostics Inc., USA) for early detection of HIV infection and reduction of false positive rate.
Methods
Four seroconversion panels were included. The third-generation ADVIA Centaur HIV 1/O/2 enhanced (EHIV) assay (Siemens Healthcare Diagnostics Inc., USA) and fourth-generation CHIV assay were used to test each panel for HIV infection. The presence of antigen was confirmed using HIV p24 antigen assay. To evaluate false-positivity and specificity, 54 HIV false-positive and HIV-negative serum samples from 100 hospitalized patients and 600 healthy subjects were included.
Results
Compared to the EHIV assay, the CHIV assay had a shorter window for three of the seroconversion panels: a difference of 10 days and two bleeds in one panel, and 4 days and one bleed in the other two panels. Only 34 of the 54 (63%) samples known to yield false-positive results by EHIV assay had repeatedly yielded reactive results in the CHIV assay. One of the 600 healthy subjects had a false-positive result with the CHIV assay; thus, the specificity was 99.85% (699/700). CHIV accurately determined the reactive results for the HIV-confirmed serum samples from known HIV patients and Korea Food & Drug Administration (KFDA) panels.
Go to : 
In 2008, HIV was estimated to cause 2.7 million new infections and 2 million deaths worldwide [1]. The rate of HIV infection is constantly increasing, with 888 new infections being reported in 2011 [2]. A recent study showed that delayed diagnosis of HIV and late presentation were major risk factors of early death in Korea [3], and 41% of the newly diagnosed HIV-infected individuals presented with advanced HIV disease [4]. Early diagnosis of HIV infection reduces morbidity and mortality and minimizes potential transmission of HIV. Recently published guidelines include a routine HIV screening test, which can benefit the patients by detecting HIV before seroconversion [5]. When patients are first exposed to HIV, serum HIV antibodies are produced; these can be detected at least 3 weeks later by third-generation antibody assays [6]. The window period, from the presence of HIV-1 RNA in plasma to antibody seroconversion, varies between 10 and 27 days, depending on the route of infection [7]. The HIV p24 antigen assay can detect p24 antigen, beginning approximately on the 15th day and peaking between 20 to 30 days [8], thus enabling early diagnosis of HIV infection. However, the p24 antigen titer continuously decreases after reaching its peak and is usually undetectable. A negative result for p24 antigen assay after antibody seroconversion does not exclude the possibility of HIV infection.
Combining both antigen and antibody detection increases sensitivity in the early seroconversion window, while improving the chances of identification of low-titer anti-HIV antibodies and persistent antigenemia in the late stage. Fourth-generation assays have been reported to possess potential non-specific reactivity, because two principles are combined in one assay [7, 9]. Early fourth-generation assays had limited ability to detect HIV antigen compared to single p24 antigen assays. The risk of interference was also predicted to be higher, because both serological markers were determined in one test well. The specificity of fourth-generation assays was reported to be lower than that of third-generation assays for screening blood donors, and the high rate of false-positive results (0.2%) resulted in difficulties in blood donor screening [9]. Newer fourth-generation HIV assays, however, have improved on the limitations of previous assays, while reducing the seroconversion window [10-12].
The new fourth-generation ADVIA Centaur HIV antigen/antibody combo (CHIV) assay (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA) was recently introduced in Korea. This assay appears to reduce the seroconversion window, comparable to the p24 antigen assay, and is thus expected to substitute for the third-generation ADVIA Centaur 1/O/2 enhanced antibody (EHIV) assay (Siemens Healthcare Diagnostics Inc.). In this study, we evaluated the performance of the prototype fourth-generation ADVIA Centaur HIV assay to accurately detect recent HIV infection and reduce the HIV seroconversion window compared to the EHIV assay.
Go to : 
This study was conducted between February and March 2012 in the Samsung Medical Center, Seoul, Korea. The study protocol was approved by Institutional Review Board of the Samsung Medical Center.
To evaluate early detection of HIV infection, four commercially available seroconversion panels (PRB 966, PRB 969, PRB 970, and PRB 971) purchased from Boston Biomedica, Inc. (BBI) Diagnostics (West Bridgewater, MA, USA) were tested.
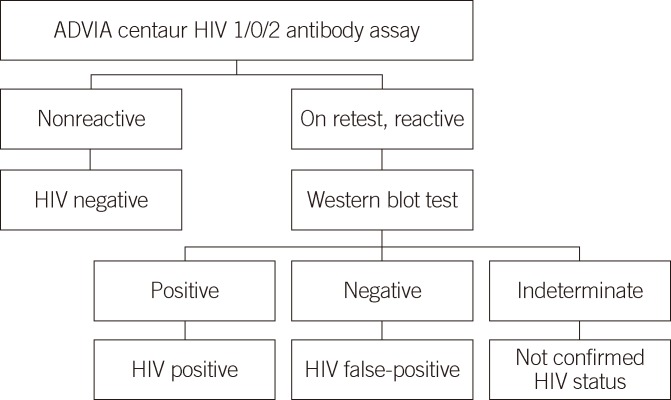
To evaluate the false-positivity and specificity of the CHIV assay, two sets of samples were tested: a batch of HIV false-positive and HIV-negative serum samples from hospitalized patients and healthy subjects who requested an HIV antibody test, and 54 HIV false-positive serum samples, which repeatedly tested reactive on EHIV assay, but produced negative results on western blot analysis (Fig. 1). Serum samples from 100 consecutive patients hospitalized in the Department of Internal Medicine and the intensive care unit of the Samsung Medical Center and 600 consecutive healthy subjects who visited the health promotion center for routine checkup were included to determine specificity. All samples from 100 hospitalized and 600 healthy subjects were classified as HIV-negative, with nonreactive results on EHIV assay. The accuracy of the CHIV assay was evaluated using 14 known HIV-positive serum samples, which repeatedly produced reactive results on EHIV assay and were confirmed HIV positive by western blot analysis (Fig. 1) and two Korea Food & Drug Administration (KFDA) standard panels (09/030 8 and 16) containing eight HIV-positive, ten indeterminate, and four negative serum samples. These known HIV-positive samples yielded both a gp160/gp120/gp41 band and at least one p24 or p31 band on western blot analysis. The 54 HIV false-positive and 14 known HIV-positive serum samples were collected from March 2010 to March 2012. Serum samples from 100 hospitalized and 600 healthy subjects were included during March 2012.
The ADVIA Centaur CHIV assay is an antigen-bridging, magnetic microparticle-based, chemiluminometric immunoassay that detects antibodies against HIV-1 group M and O, HIV-2, and p24 antigens in the serum or plasma. The ADVIA Centaur CHIV assay uses recombinant antigens, including an HIV-1 envelope protein (gp41/120) and an HIV-2 envelope protein (gp36), and three monoclonal antibodies specific to HIV p24 antigen. A synthetic peptide is added to detect antibodies to the HIV-1 group O. A direct relationship exists between the amount of HIV antibody and/or p24 antigen in the serum and the amount of relative light units (RLU) detected by the ADVIA Centaur system. A reactive or nonreactive result was determined according to the index value of signal to cut off (S/CO) ratio established with calibrators. An S/CO ratio of ≥1.0 was considered reactive and that of <1.0 was nonreactive. Reactive serum samples had an S/CO ratio of 1.0 to >2.0 in our system. All initially reactive serum samples were retested in duplicate after centrifugation at 10,000×g for 10 min. If the retest result was reactive, the sample was sent for western blot analysis. Samples with positive results for western blot analysis were considered HIV positive.
The ADVIA Centaur EHIV assay is a microparticle-based, chemiluminometric immunoassay that detects antibodies to HIV-1 group M and O and HIV-2. This assay contains a complex of streptavidin-coated microparticles and acridinium-labeled HIV-1 and HIV-2 recombinant antigens and peptides. A reactive or nonreactive result was determined according to the index value of the S/CO ratio established with calibrators. An S/CO ratio of ≥1.0 was considered reactive and <1.0 was nonreactive. Reactive serum samples had an S/CO ratio of 1.0 to >50.0 in our system. All initially reactive serum samples were retested and subjected to western blot analysis, as described above.
Elecsys HIV p24 antigen assay (Roche Diagnostics GmbH, Mannheim, Germany) is an electrochemiluminescence immunoassay that determines the concentration of HIV-1 p24 antigen. This assay contains a biotinylated monoclonal HIV p24 antibody and a ruthenium-labeled monoclonal HIV p24 antibody that form a sandwich with streptavidin-coated microparticles. The Elecsys software compares the electro-chemiluminescence signal with cut-off values established by HIV antigen calibrators and determines a positive result when the S/CO ratio is >1.0. A sample with S/CO ratio of ≥1.0 was considered HIV positive.
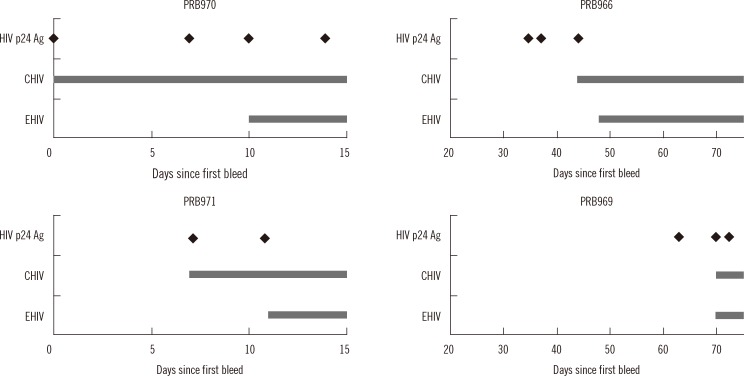
The relative performance of early HIV detection with seroconversion panels was determined by comparing the results obtained with CHIV, EHIV, and HIV p24 antigen assays. The seroconversion day on each panel was designated as the first day since the first bleed that a positive result was obtained.
To analyze the false-positive results of the ADVIA Centaur CHIV assay, we reviewed clinical information from 54 subjects with false-positive results in electronic medical records and described possible factors known to cause false-positive results. The specificity of the CHIV assay with a 95% confidence interval was determined for serum samples from 100 hospitalized and 600 healthy subjects. Statistical analyses were performed using MedCalc Statistical Software (version 11.5.1; MedCalc Software, Mariakerke, Belgium).
Go to : 
Compared to the EHIV assay, the ADVIA Centaur CHIV assay had a shorter window period for three panels: a difference of 10 days and two bleeds for PPB970 and four days and one bleed for PPB966 and PPB971. In the PPB969 panel, which includes samples from 60 days after the first bleed, no difference was observed between EHIV and CHIV in the detection of HIV infection. Compared to the HIV p24 antigen assay, the CHIV assay had the same seroconversion day in the PPB970 and PPB971 panels, and delayed detection in the PPB966 and PPB969 panels (Fig. 2).
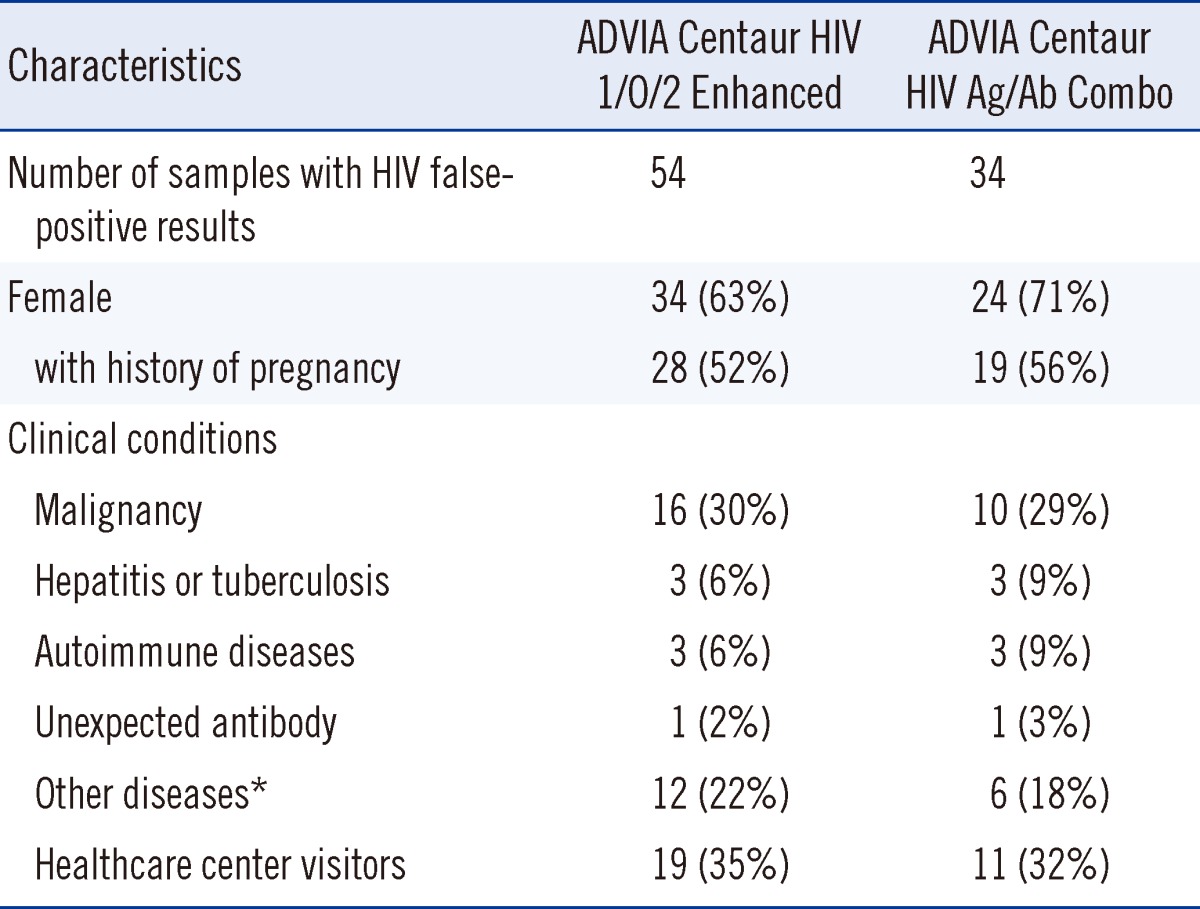
The ADVIA Centaur CHIV assay had 34 HIV false-positive results (63%) among the 54 known false-positive serum samples. The median S/CO ratio was 1.4 (range, 1.0 to >12.0) among the 34 HIV false-positive results. Two false-positive serum samples had S/CO ratios of >12.0 on the CHIV assay, which were calculated as 12.0 in our statistical analysis. Patients with HIV false-positive results had various clinical conditions, including malignant neoplasms, hepatitis, tuberculosis, autoimmune diseases (Sjogren's syndrome, polychondritis, and rheumatoid arthritis), multiple pregnancies, unexpected anti-le(a) antibody, recent rickettisial infection, and benign mass. The comparison of the clinical conditions of subjects with HIV false-positive results for EHIV and CHIV assays is summarized in Table 1.
The specificity of the CHIV assay was 99.85% (699/700, 95% confidence interval [CI]: 99.21%-100%) for the 100 hospitalized patients and 600 healthy subjects. The specificity of EHIV assay was 100% (95% CI: 99.47%-100%). We did not obtain any reactive results for the 100 hospitalized patients by using the CHIV assay, but four of the 600 healthy subjects were initially reactive (three of the four subjects were nonreactive on repeating the test). One healthy subject who tested negative on EHIV assay consistently had an S/CO ratio of >1.0 (initial S/CO ratio of 1.04 and retest S/CO ratios of 1.06/1.09/1.04) on CHIV assay.
The CHIV assay accurately determined the presence of HIV infection in all 14 known HIV-positive serum samples from patients and eight HIV-1-positive samples from the two KFDA standard panels. All CHIV assay results for the KFDA panels were concordant with the EHIV assay results: all four HIV-negative serum samples from the KFDA standard panels tested negative, nine of 10 indeterminate samples tested positive, and the one indeterminate serum sample tested negative by the CHIV, EHIV, and HIV p24 antigen assays.
Go to : 
The purpose of this study was to evaluate a new fourth-generation CHIV assay focusing on early diagnosis of HIV infection by reducing the seroconversion window and false-positive rate. Detecting recent infection by HIV is the strength of the fourth-generation HIV assays, compared to the third-generation assays. According to previous studies, which used seroconversion panels and HIV assays, the fourth-generation combo assays have reduced the diagnostic window compared to the third-generation assays up to 5 days [13], 1 week [10, 14], or 26 days [15]; this varied across the studies. In this study, the HIV seroconversion window of the CHIV assay was reduced in three of four seroconversion panels (reduction for 4 days, 4 days and 10 days, respectively) and was close to that of the Elecsys HIV p24 antigen assay in two seroconversion panels, while there was a longer window in two other panels, which can be attributed to delayed HIV antibody production.
Detecting both HIV antibodies and p24 antigen simultaneously, previous fourth-generation combo assays had potential weak points of non-specific reactivity and false-positive results with possible low specificity. Newer fourth-generation HIV assays should be validated for the known limitation of previous assays. According to the recent studies [16, 17], the average specificity of the fourth-generation assay was from 99.5% to 99.9%, which was equivalent to that of the third-generation assays.
In our study involving 700 samples, including 100 hospital patients with clinical conditions that can lead to false-positive results and 54 EHIV-positive samples from various clinical conditions, we obtained an excellent specificity of 99.85% and noticed the potential to reduce the rate of false-positive results in HIV screening. The specificity of 99.76% in blood donors and 99.2% among hospitalized patients was reported in a study for evaluation of the ADVIA Centaur CHIV assay [18]. False-positive results can occur during HIV screening because of various clinical conditions, such as recent influenza or hepatitis B vaccinations, viral infections, autoimmune diseases, renal failure, blood transfusion, liver disease, hemodialysis, multiple pregnancies, or pregnancy at a young age [6, 19, 20]. Considering that a false-positive result requires a repeat test and confirmatory western blot analysis [21], a low false-positive rate reduces the labor, cost, and time needed to perform the required tests. According to our results, this newer fourth-generation HIV assay seemed to improve on the limitations and reduced the seroconversion window compared to the third-generation assays.
The analytical sensitivity of the CHIV assay provided by the manufacturer was 1.15 IU/mL with HIV-1 p24 antigen 1st international reference reagent (90/636). The p24 antigen, CHIV, and other combo assays should meet the analytical sensitivity at least 2 IU/mL using WHO standard, for approval of HIV combo assays in the Conformite Europeenne [22]. The newer fourth-generation HIV assays showed 1.13 IU/mL of lowest detection limit using HIV p24 antigen sensitivity panel [17]. Although we could not evaluate the analytical sensitivity of the ADVIA Centaur CHIV assay in this study, we may expect that it has the comparable sensitivity with other fourth-generation HIV assays.
There was inherent bias in estimating the false-positive rate because of the retrospective nature of the HIV false-positive samples and the limited sample size. A prospective study of a larger scale may be necessary to evaluate the efficiency of reducing the false-positive rate in a tertiary hospital setting.
In conclusion, our study showed that the fourth-generation ADVIA Centaur CHIV assay was sensitive and specific for HIV seroconversion panels, HIV false-positive serum samples, and samples from HIV-negative hospitalized subjects. The ADVIA Centaur CHIV assay shortened the serological window, and therefore, may be useful to reduce morbidity and mortality associated with HIV infection, and thereby, the risk of HIV transmission.
Go to : 
Acknowledgements
We would like to thank HY Shin, M.T. and PH Kim, M.T. for their technical assistance.
Go to : 
References
1. UNAIDS. AIDS epidemic update 2009. Geneva: UNAIDS/WHO;2009.
2. Korea Centers for Disease Control and Prevention. Analysis of HIV/AIDS Notifications in Korea. Updated on Jul 2013. http://www.cdc.go.kr/CDC/notice/CdcKrTogether0302.jsp?menuIds=HOME001-MNU0004-MNU0085-MNU0088&cid=21276.
3. Lee SH, Kim KH, Lee SG, Chen DH, Jung DS, Moon CS, et al. Trends of mortality and cause of death among HIV-infected patients in Korea, 1990-2011. J Korean Med Sci. 2013; 28:67–73. PMID: 23341714.

4. Choe PG, Park WB, Song JS, Kim NH, Park JY, Song KH, et al. Late presentation of HIV disease and its associated factors among newly diagnosed patients before and after abolition of a government policy of mass mandatory screening. J Infect. 2011; 63:60–65. PMID: 21621848.

5. Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006; 55:1–17. quiz CE1-4. PMID: 16988643.
6. Mahajan VS, Pace CA, Jarolim P. Interpretation of HIV serologic testing results. Clin Chem. 2010; 56:1523–1526. PMID: 20876778.

7. Gürtler L, Mühlbacher A, Michl U, Hofmann H, Paggi GG, Bossi V, et al. Reduction of the diagnostic window with a new combined p24 antigen and human immunodeficiency virus antibody screening assay. J Virol Methods. 1998; 75:27–38. PMID: 9820572.

8. Busch MP, Satten GA. Time course of viremia and antibody seroconversion following human immunodeficiency virus exposure. Am J Med. 1997; 102:117–124. discussion 125-6. PMID: 9845513.

9. Weber B, Fall EH, Berger A, Doerr HW. Reduction of diagnostic window by new fourth-generation human immunodeficiency virus screening assays. J Clin Microbiol. 1998; 36:2235–2239. PMID: 9665998.

10. Brust S, Duttmann H, Feldner J, Gürtler L, Thorstensson R, Simon F. Shortening of the diagnostic window with a new combined HIV p24 antigen and anti-HIV-1/2/O screening test. J Virol Methods. 2000; 90:153–165. PMID: 11064116.

11. Weber B, Gürtler L, Thorstensson R, Michl U, Mühlbacher A, Bürgisser P, et al. Multicenter evaluation of a new automated fourth-generation human immunodeficiency virus screening assay with a sensitive antigen detection module and high specificity. J Clin Microbiol. 2002; 40:1938–1946. PMID: 12037046.

12. Beelaert G, Fransen K. Evaluation of a rapid and simple fourth-generation HIV screening assay for qualitative detection of HIV p24 antigen and/or antibodies to HIV-1 and HIV-2. J Virol Methods. 2010; 168:218–222. PMID: 20561542.

13. Weber B, Orazi B, Raineri A, Thorstensson R, Bürgisser P, Mühlbacher A, et al. Multicenter evaluation of a new 4th generation HIV screening assay Elecsys HIV combi. Clin Lab. 2006; 52:463–473. PMID: 17078473.
14. Kang HJ, Yoo KH, Kim HS, Cho HC. Evaluation of Abbott fourth generation HIV antigen and Antibody assays. Korean J Lab Med. 2006; 26:39–44. PMID: 18156698.

15. Kwon JA, Yoon SY, Lee CK, Lim CS, Lee KN, Sung HJ, et al. Performance evaluation of three automated human immunodeficiency virus antigen-antibody combination immunoassays. J Virol Methods. 2006; 133:20–26. PMID: 16313975.

16. Butto S, Suligoi B, Fanales-Belasio E, Raimondo M. Laboratory diagnostics for HIV infection. Ann Ist Super Sanita. 2010; 46:24–33. PMID: 20348616.

17. Song EY, Hur M, Roh EY, Park MH, Moon HW, Yun YM. Performances of four fourth-generation human immunodeficiency virus-1 screening assays. J Med Virol. 2012; 84:1884–1888. PMID: 23080491.

18. Pumarola T, Freeman J, Saxton E, Dillon P, Bal T, van Helden J. Performance evaluation of the ADVIA Centaur(®) HIV Ag/Ab Combo assay. J Virol Methods. 2010; 170:16–20. PMID: 20723562.

19. Erickson CP, McNiff T, Klausner JD. Influenza vaccination and false positive HIV results. N Engl J Med. 2006; 354:1422–1423. PMID: 16571889.

20. Chao TT, Sheffield JS, Wendel GD Jr, Ansari MQ, McIntire DD, Roberts SW. Risk factors associated with false positive HIV test results in a low-risk urban obstetric population. J Pregnancy. 2012; 2012:841979. PMID: 21860798.

21. Kasper DL, editor. Harrison's principles of internal medicine. 18th ed. New York: McGraw-Hill;2012.
22. Miedouge M, Grèze M, Bailly A, Izopet J. Analytical sensitivity of four HIV combined antigen/antibody assays using the p24 WHO standard. J Clin Virol. 2011; 50:57–60. PMID: 20926337.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download