1. Feld LG, Meyers KE, Kaplan BS, Stapleton FB. Limited evaluation of microscopic hematuria in pediatrics. Pediatrics. 1998; 102:E42. PMID:
9755279.

2. Vachvanichsanong P, Malagon M, Moore ES. Recurrent abdominal and flank pain in children with idiopathic hypercalciuria. Acta Paediatr. 2001; 90:643–648. PMID:
11440097.

3. Zerwekh JE. Bone disease and hypercalciuria in children. Pediatr Nephrol. 2010; 25:395–401. PMID:
19885683.

4. Pace G, Aceto G, Cormio L, Traficante A, Tempesta A, Lospalluti ML, et al. Nocturnal enuresis can be caused by absorptive hypercalciuria. Scand J Urol Nephrol. 1999; 33:111–114. PMID:
10360451.
5. Kaneko K, Tsuchiya K, Kawamura R, Ohtomo Y, Shimizu T, Yamashiro Y, et al. Low prevalence of hypercalciuria in Japanese children. Nephron. 2002; 91:439–443. PMID:
12119474.

6. Kaneko K, Chiba M, Hashizume M, Kunii O, Sasaki S, Shimoda T, et al. Extremely high prevalence of hypercalciuria in children living in the Aral Sea region. Acta Paediatr. 2002; 91:1116–1120. PMID:
12434899.

7. Ryu KH, Lee SJ, Lee K, Jung JS. Idiopathic hypercalciuria in children. J Korean Pediatr Soc. 1989; 32:809–815.
8. Ahn YH, Kim KH, Ko CW, Koo JH. Idiopathic hypercalciuria in children. Korean J Nephrol. 1989; 8:79–84.
9. Ghazali S, Barratt TM. Urinary excretion of calcium and magnesium in children. Arch Dis Child. 1974; 49:97–101. PMID:
4406087.

10. Arrabal-Polo MA, Arias-Santiago S, Girón-Prieto MS, Abad-Menor F, López-Carmona Pintado F, Zuluaga-Gomez A, et al. Hypercalciuria, hyperoxaluria, and hypocitraturia screening from random urine samples in patients with calcium lithiasis. Urol Res. 2012; 40:511–515. PMID:
22484727.

11. Koyun M, Güven AG, Filiz S, Akman S, Akbas H, Baysal YE, et al. Screening for hypercalciuria in schoolchildren: what should be the criteria for diagnosis? Pediatr Nephrol. 2007; 22:1297–1301. PMID:
17549524.

12. Hong YH, Dublin N, Razack AH, Mohd MA, Husain R. Twenty-four hour and spot urine metabolic evaluations: correlations versus agreements. Urology. 2010; 75:1294–1298. PMID:
19914693.

13. Matos V, van Melle G, Boulat O, Markert M, Bachmann C, Guignard JP. Urinary phosphate/creatinine, calcium/creatinine, and magnesium/creatinine ratios in a healthy pediatric population. J Pediatr. 1997; 131:252–257. PMID:
9290612.

14. Safarinejad MR. Urinary mineral excretion in healthy Iranian children. Pediatr Nephrol. 2003; 18:140–144. PMID:
12579403.

15. Sönmez F, Akcanal B, Altincik A, Yenisey C. Urinary calcium excretion in healthy Turkish children. Int Urol Nephrol. 2007; 39:917–922. PMID:
17043921.

16. Ahn HS, Suh BK, Seo JK, Oh SH, Lee SI, Lee C, editors. Textbook of Pediatrics. 10th ed. Seoul: Miraen Publishing Co.;2012. p. 916–917.
17. Mir S, Serdaroqlu E. Quantification of hypercalciuria with the urine calcium osmolality ratio in children. Pediatr Nephrol. 2005; 20:1562–1565. PMID:
16133062.

18. Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987; 317:1098. PMID:
3657876.

19. Reusz GS, Dobos M, Byrd D, Sallay P, Miltenyi M, Tulassay T. Urinary calcium and oxalate excretion in children. Pediatr Nephrol. 1995; 9:39–44. PMID:
7742220.

20. Alconcher LF, Castro C, Quintana D, Abt N, Moran L, Gonzalez L, et al. Urinary calcium excretion in healthy school children. Pediatr Nephrol. 1997; 11:186–188. PMID:
9090660.

21. Esbjörner E, Jones IL. Urinary calcium excretion in Swedish children. Acta Paediatr. 1995; 84:156–159. PMID:
7756801.
22. Sargent JD, Stukel TA, Kresel J, Klein RZ. Normal values for random urinary calcium to creatinine ratios in infancy. J Pediatr. 1993; 123:393–397. PMID:
8355114.

23. Remer T, Neubert A, Maser-Gluth C. Anthropometry-based reference values for 24-hr urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am J Clin Nutr. 2002; 75:561–569. PMID:
11864864.
24. Mori Y, Hiraoka M, Suganuma N, Tsukahara H, Yoshida H, Mayumi M. Urinary creatinine excretion and protein/creatinine ratios vary by body size and gender in children. Pediatr Nephrol. 2006; 21:683–687. PMID:
16550362.

25. Manz F, Kehrt R, Lausen B, Merkel A. Urinary calcium excretion in healthy children and adolescents. Pediatr Nephrol. 1999; 13:894–899. PMID:
10603144.

26. Seifert-McLean CM, Cromer BA, Mosher G, Mahan JD. Urinary calcium excretion in healthy adolescents. J Adolesc Health Care. 1989; 10:300–304. PMID:
2732110.

27. Metz MP. Determining urinary calcium/creatinine cut-offs for the paediatric population using published data. Ann Clin Biochem. 2006; 43:398–401. PMID:
17022882.

28. Slev PR, Bunker AM, Owen WE, Roberts WL. Pediatric reference intervals for random urine calcium, phosphorus and total protein. Pediatr Nephrol. 2010; 25:1707–1710. PMID:
20473690.

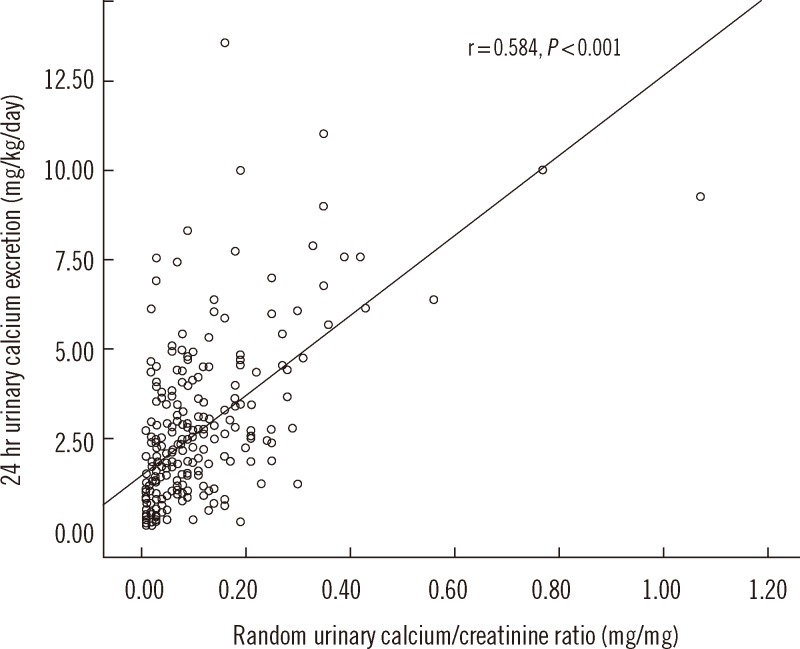
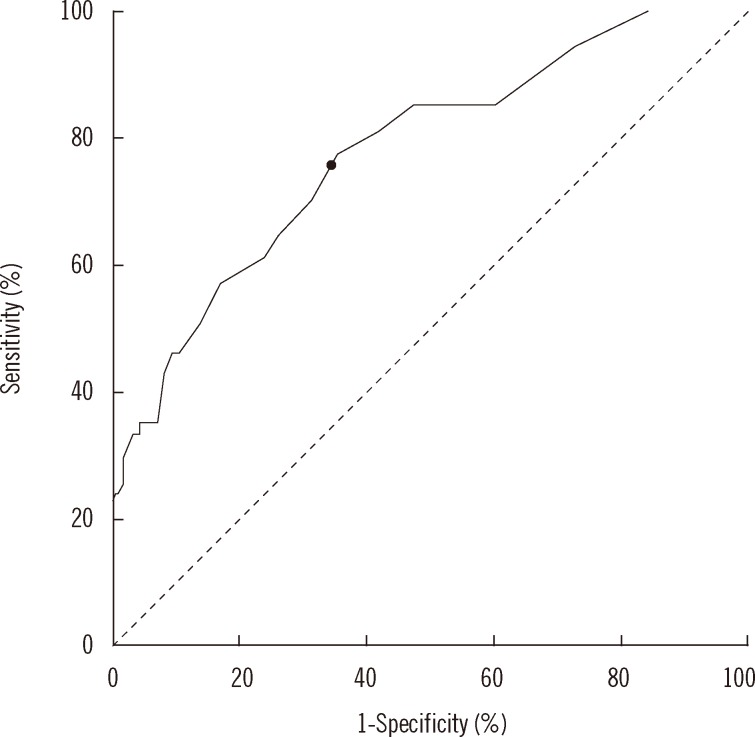




 PDF
PDF ePub
ePub Citation
Citation Print
Print


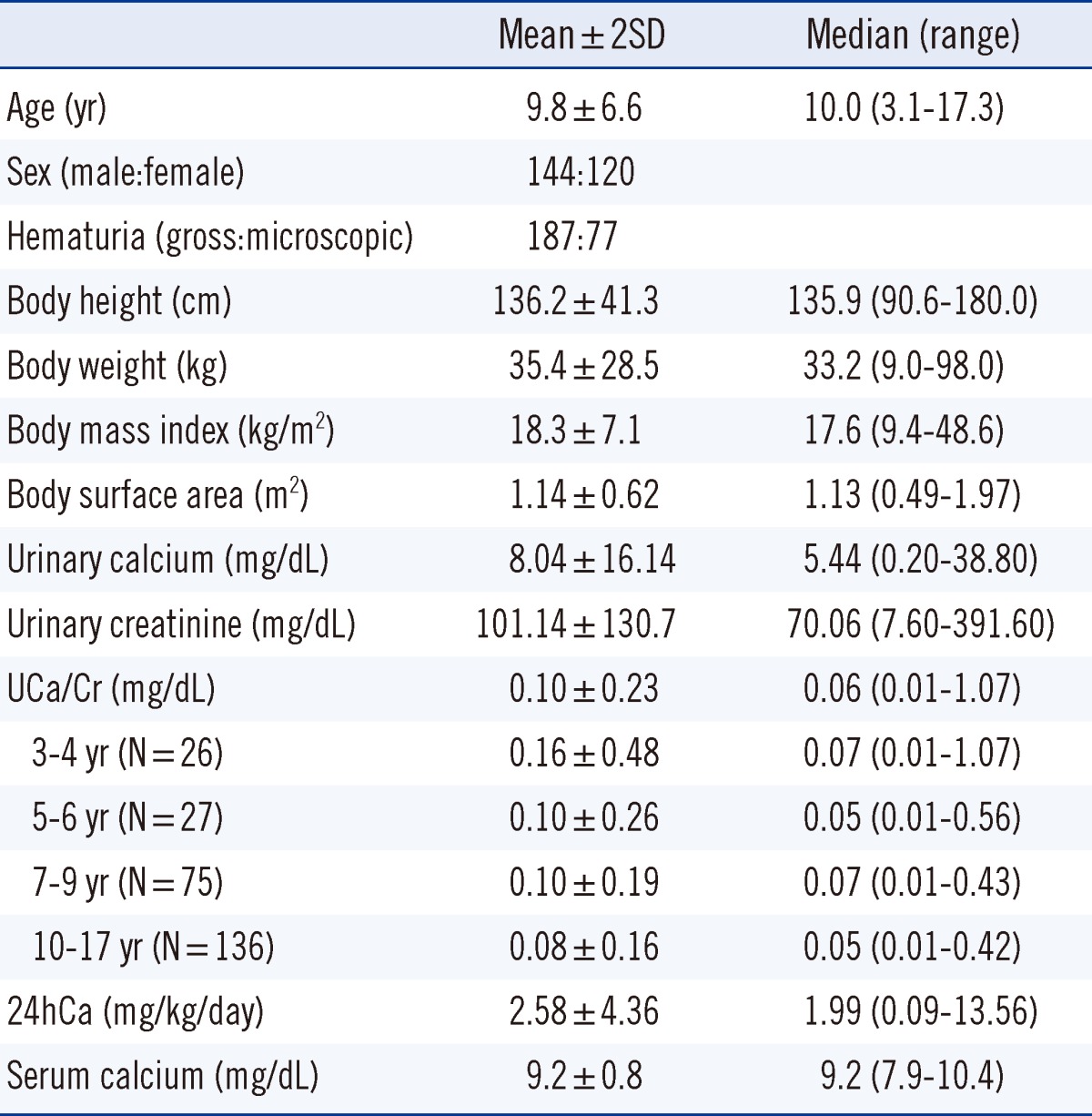
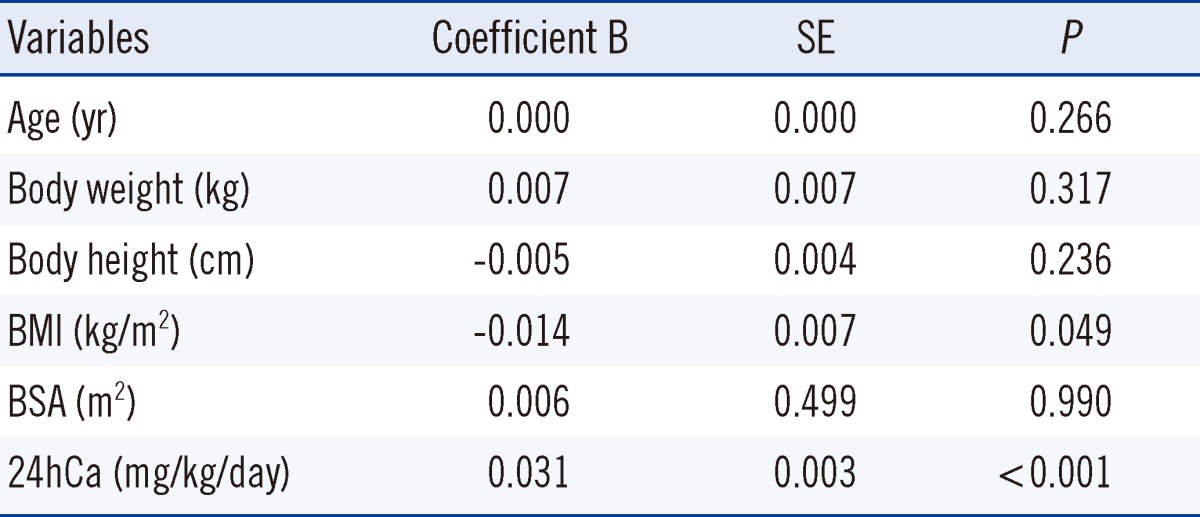
 XML Download
XML Download