Abstract
Background
Group B streptococcus (GBS) infection is a leading cause of neonatal morbidity and mortality worldwide. Here, we present the analytical and diagnostic usefulness of a new real-time PCR-based assay (Xpert GBS; Cepheid, USA) for rapid and accurate prenatal GBS screening.
Methods
We enrolled 175 pregnant women who were between 35 and 39 weeks of gestation. The analytical performance of the Xpert GBS assay was first tested using a reference GBS strain. Next, to test diagnostic performance, rectovaginal swabs were obtained from pregnant women who visited the hospital for regular antenatal screening after 34 weeks of gestation. The results of the Xpert GBS assay were compared to those of standard culture for the detection of prenatal GBS colonization.
Results
When any positive result from Xpert GBS or culture was considered a true positive, the sensitivity of the Xpert GBS assay and culture were 91% (20/22; 95% CI [confidence interval], 72-98) and 68% (15/22; 95% CI, 47-84), respectively. The specificity of both methods was 100% (153/153; 95% CI, 97-100). The sensitivity and specificity of the Xpert GBS assay, using the culture results as a reference, were 86.7% and 95.6%, respectively. In the Xpert GBS assay, the median threshold cycle of vaginally colonized samples was significantly lower than rectally colonized samples (P<0.01).
Group B streptococcus (GBS) infection causes neonatal sepsis and postpartum infection. In Korea, the incidence of GBS infection in newborns is much lower than in other countries because of the low prevalence of GBS colonization in pregnant women [1-4].
However, these rates vary widely between socioeconomic and ethnic groups [5]. In Korea, the GBS colonization rate among pregnant women was estimated to be about 8%, which resulted in physicians' diminished attention to the CDC (Centers for Disease Control and Prevention) recommendations for universal GBS screening [2, 6, 7]. However, the most recently reported rate, at approximately 8%, is higher than that reported 10 yr ago (2.61%) [8], although the longitudinal change in this rate has not been followed well in Korea. In addition, GBS is a leading cause of neonatal sepsis and meningitis [9]. For these reasons, prenatal GBS screening will be more important in Korean in the future.
The current gold standard GBS detection method is incubation of a specimen in selective medium followed by subculture on a blood agar plate [10]. However, the sensitivity of this standard culture method for the detection of GBS colonization is only 54-87% [11, 12]. It has long turnaround time, and requires up to 36-72 hr before the results can be reported [10, 11]. It also requires an experienced technician to identify non-beta-hemolytic GBS. To overcome these problems, more rapid and sensitive techniques have been developed, including DNA probe and nucleic acid amplification tests (NAAT) [13, 14]. Ke et al. [15] developed an RT-PCR method based on amplification of a cfb gene fragment that is present in virtually every strain of GBS.
In this study, we demonstrated the analytical and diagnostic performance of an RT-PCR assay that amplifies the cfb gene (Xpert GBS; Cepheid, Sunnyvale, CA, USA) and compared it to the standard culture method in the setting of prenatal GBS screening. In addition, we compared the threshold cycle (CT) values of the samples from the subjects to clarify the quantitative difference in GBS according to colonization site.
We enrolled 175 pregnant women who visited our hospital for regular prenatal care after 34 weeks of gestation. This study was approved by the research ethics committees of Seoul National University Hospital and Cheil General Hospital, and all the study subjects gave written informed consent.
All specimens were collected with a Copan Venturi Transystem collection device (Copan innovation, Corona, CA, USA), which consists of a pair of rayon swabs and liquid Stuart transport medium. For standard culture, separate vaginal and rectal specimens were obtained; for the Xpert GBS assay, a single, combined, rectovaginal specimen was obtained. A vaginal specimen was obtained from the mucosa of the lower third of the vagina after excessive secretions or discharge was wiped away. A rectal specimen was obtained from the rectal mucosa at approximately 2.5 cm beyond the anal sphincter. For a combined rectovaginal specimen, a swab was first inserted into the vagina and then into the anus. All specimens were tested within 24 hr after collection.
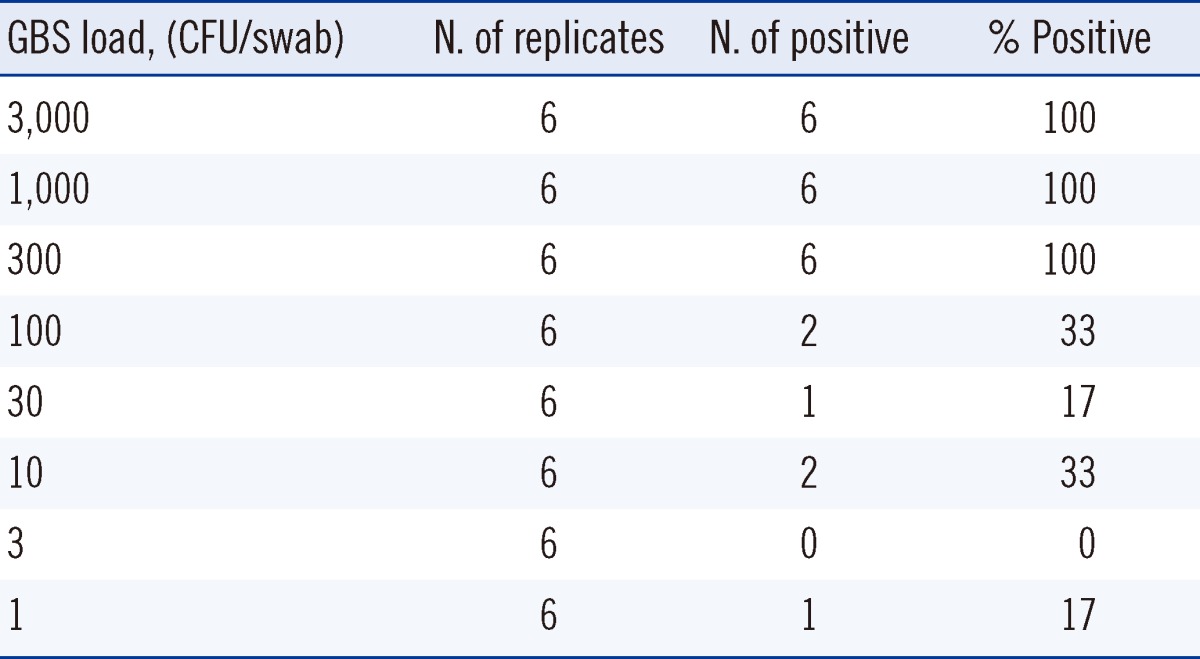
The limit of detection was determined by probit analysis [16]. Suspensions of Streptococcus agalactiae (American Type Culture Collection 27956) were prepared as serial dilutions of 3,000, 1,000, 300, 100, 30, 10, 3, and 1 colony forming unit (CFU)/swab. Each dilution was analyzed with 6 replicates.
Analytical specificity was determined by testing isolated bacterial colonies from cell suspension-soaked swabs. The bacteria tested were as follows: a mixed fecal flora (Bacteroides fragilis, Lactobacillus, Clostridium perfringens, Escherichia coli, Klebsiella oxytoca, and Enterobacter aerogenes), a mixed urogenital flora (Staphylococcus epidermidis, Enterococcus faecalis, Escherichia coli, Corynebacterium, and Candida albicans), Streptococcus bovis, Streptococcus pyogenes, Streptococcus viridans group, Streptococcus anginosus group, Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus, methicillin-sensitive S. aureus, β-hemolytic Enterococcus, vancomycin-resistant Enterococcus, Enterococcus faecalis, and coagulase-negative Staphylococcus.
To determine the reproducibility of intra- and inter-Xpert GBS assay measurements, a S. agalactiae suspension (550 CFU/swab) was examined 6 times a day (intra-assay) and on 4 consecutive days (inter-assay). The swabs were produced by adding 100 µL of a bacterial cell suspension containing 5,500 CFU/mL daily just before testing.
The diagnostic usefulness of the Xpert GBS assay was evaluated in comparison to the standard culture method. For each sample, laboratory turnaround time (TAT; the time from receipt to results reporting) was recorded. For the Xpert GBS assay, a sample swab and two reagent solutions are placed into appropriate chambers of Xpert GBS cartridge, which is then loaded into a GeneXpert Dx module to complete sample preparation, target amplification, and product detection. The invalid or error results in Xpert GBS assay are retested until positive or negative results are obtained.
For the culture method, swab specimens were inoculated into a selective broth medium that consisted of Todd-Hewitt broth supplemented with gentamicin (8 µg/mL) and nalidixic acid (15 µg/mL) [17]. Inoculation was followed by overnight incubation and subculture on 5% sheep blood agar for 18-24 hr at 35℃ in an atmosphere containing 5% CO2. Specific identification of colonies suggestive of GBS was initially evaluated using a Vitek-2 apparatus (bioMérieux, Hazelwood, MO, USA) with additional tests, and then stored at -80℃ in 10% skim milk. Additionally, a commercial slide agglutination kit (Seroiden Strepto Kit; Eiken, Tokyo, Japan) was used to determine Lancefield group. If GBS was not identified after incubation for 18-24 hr on a sheep blood agar plate, the plate was reincubated and reexamined at 48 hr.
The lowest concentration at which all replicates yielded positive results in the Xpert GBS assay was 300 CFU/swab (Table 1). The limit of detection as determined by probit analysis was 270 CFU/swab (95% confidence interval [CI], 170-810). In intra-assay analysis using samples with 550 CFU/swab, the mean CT was 38.3±1.6 with CV of 4.2%. In inter-assay analysis, the mean CT was 38.6±0.9 with CV of 2.2%. No cross-reactivity was observed among 22 bacterial strains representing 19 different species.
The mean TAT was 19.3±10.2 hr (range, 3-48). Among all 175 participants, the results for 47 (27%) could be reported the same day, the results for 120 (69%) could be reported the next day, and the results for 8 (4%) could be reported on the following day.
Among all 175 pregnant women, 22 (13%) were identified as GBS carriers, based on a positive culture and/or Xpert GBS results. Of the 22 carriers, 13 (59%) were positive by both Xpert GBS assay and culture, and 7 (32%) were positive by Xpert GBS assay and negative by repeated culture using archived swabs, while the remaining 2 (9%) were positive only by culture. These 2 were positive by repeated Xpert GBS assay using stored frozen isolates (Table 2). When considering any positive result from culture and/or the Xpert GBS assay as a true positive, the sensitivity of culture and the Xpert GBS assays were 68% (15/22; 95% CI, 47-84) and 91% (20/22; 95% CI, 72-98), respectively. Analysis of the specificity of culture and the Xpert GBS assay was meaningless.
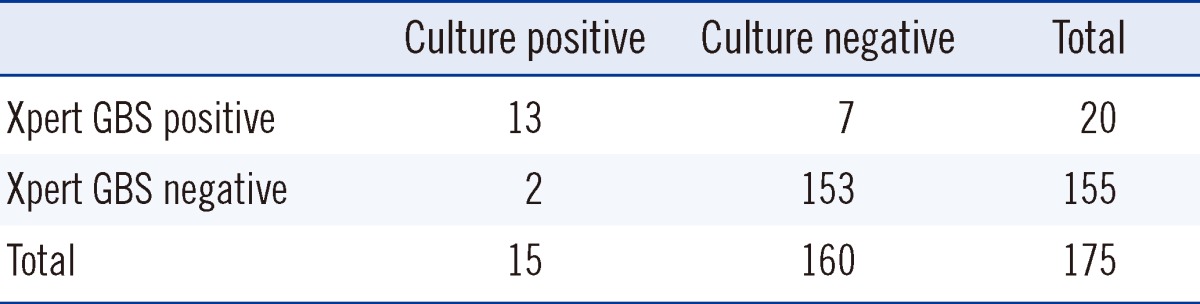
Using the culture results as a reference, the Xpert GBS assay had a sensitivity of 86.7% (13/15), a specificity of 95.6% (153/160), a positive predictive value of 65.0% (13/20), and a negative predictive value of 98.7% (153/155; Table 3).
In initial testing, no Xpert GBS results were obtained in 34 of the 175 (19%) cases. Of these cases, 24 were invalid, and 10 were errors. After retesting, 2 results were confirmed as positive and 32 results were confirmed as negative.
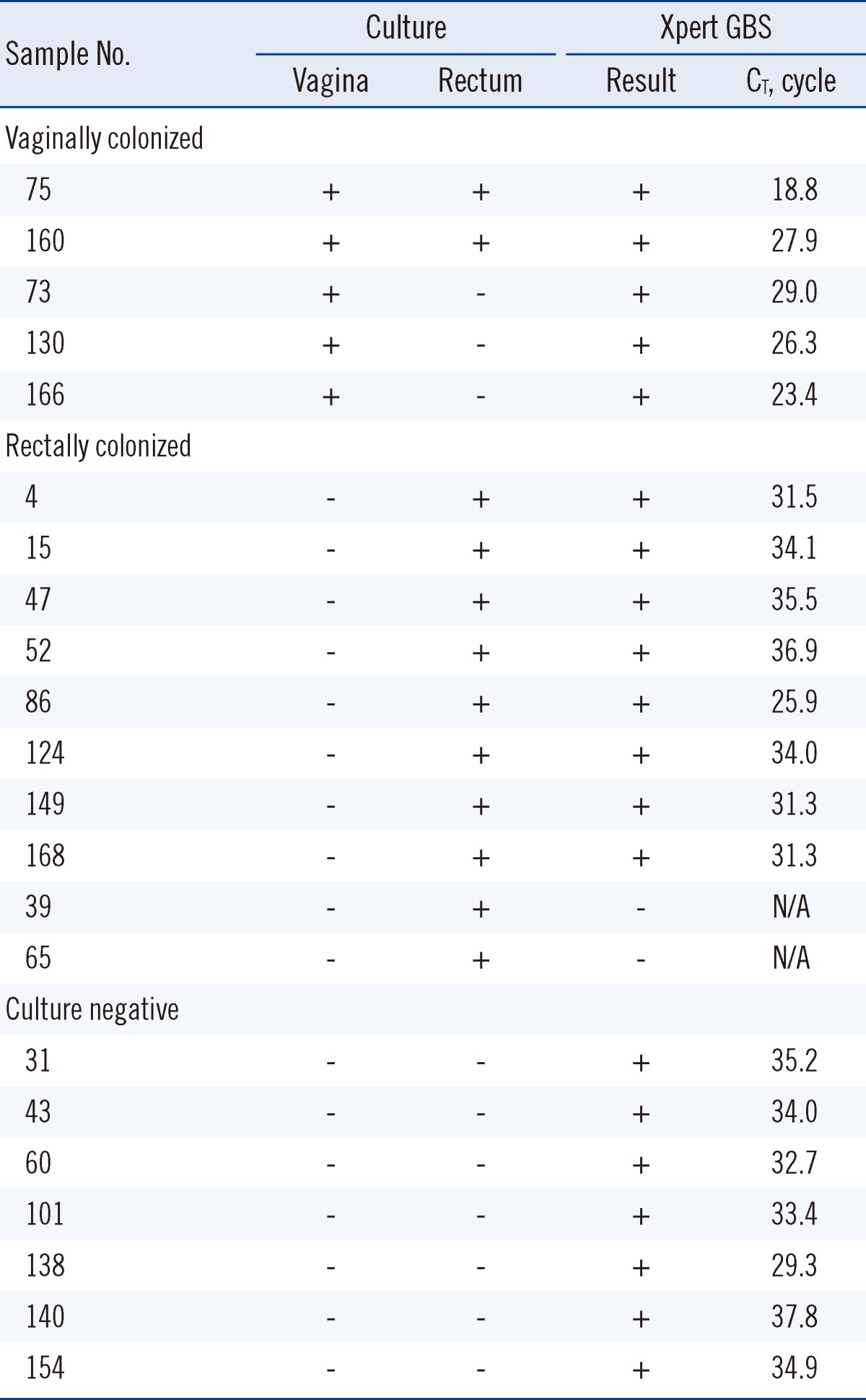
Regarding the source of the 15 culture positive results, 5 (33%) were vaginally colonized and 10 (67%) were rectally colonized (Table 4). The median CT of samples that were vaginally colonized was significantly lower than that of samples that were rectally colonized: 26.3 (interquartile range [IQR], 21.2-28.5) vs. 32.8 (IQR, 31.3-35.2; P=0.001). The median CT of the 7 culture negative and Xpert GBS positive cases was 34.0 (IQR, 32.7-35.2). The difference in the median CT between the vaginally colonized subjects and culture-negative subjects was significant (P=0.003). The difference of the median CT between the rectally colonized subjects and culture-negative subjects was not significant (P>0.05).
The number of culture positive specimens from the rectum outnumbered those from the vagina by about 2 times. Previous studies reported higher carriage rates in the rectum than in the vagina, which suggests that the gastrointestinal tract is the primary GBS reservoir, and that vaginal colonization represents dissemination from this gastrointestinal source [18, 19]. However, the vaginal GBS colonizers do not always also colonize the rectum [20]. This finding demonstrates the complex pattern of GBS colonization; therefore, to obtain the highest detection rate of GBS colonization in prenatal screening, a combined rectovaginal swab is the sample of choice.
In this study, the median CT of the vaginally colonized subjects was significantly lower than that of the non-vaginally colonized or culture negative cases. This means the vaginally colonized subjects were heavily colonized with GBS. This is the first study to demonstrate the difference in bacterial load between those who are vaginally colonized and those who are rectally colonized using RT-PCR. A previous study reported a tendency toward detection of heavier colonization in vaginal swab samples, with increasing colonization of the corresponding anal-rectal swab samples by semi-quantitative analysis [21]. The intensity of maternal GBS colonization is known to be a risk factor for recurrent colonization and vertical transmission of GBS [22, 23]. However, previous data on whether there is a differential neonatal sepsis rate from GBS colonization of the vagina compared to GBS colonization of the rectum are lacking. Further study will be needed to determine this relationship.
We retested samples with discrepant results between the Xpert GBS assay and standard culture to determine which test report ed the true result. In the 2 cases that were Xpert GBS ne gative and culture positive, retesting of stored frozen isolates by Xpert GBS assay yielded positive results. In these cases, low bacterial loads in the initial specimen that were below the limit of detection may have caused the initial false results. A previous study reported a clear association between low GBS numbers in specimens shown to contain GBS by culture and a negative PCR result [24]. In 7 cases that were Xpert GBS positive and culture negative, retesting of the archived swabs by culture showed heavy growth of enterococci colonies on the blood agar plate. It is highly possible that the lack of selectivity of the enrichment broth, particularly against enterococci, hindered the isolation of GBS. In fact, this phenomenon has been well documented by others [25]. Another possibility is detection of the cfb gene in nonviable GBS using the Xpert GBS assay in participants that might have recently received antimicrobial chemotherapy.
In this study, the prenatal GBS colonization rate of pregnant women was 13% (22/175), which is the highest rate ever report ed in a Korean population [6, 7]. This high rate can be explained by the use of the more sensitive Xpert GBS assay because the colonization rate in this study by the culture method (15/175, 8.6%) is comparable to the previously reported rate (8.3%), which was based on the standard culture method [7]. Although the false positivity of the Xpert GBS assay could not be ruled out, this finding might indicate that the sensitivity of the Xpert GBS assay surpasses that of standard culture for GBS detection. To date, 3 studies that evaluated the Xpert GBS assay have been published in peer-reviewed journals [26-28]. These studies reported that the sensitivity and specificity of intrapartum Xpert GBS assay compared to intrapartum culture were 95.8-98.5% and 64.5-99.6%, respectively [27, 28].
Interestingly, initial Xpert GBS results were not available for 34 (19%) cases. This invalid (24 cases, 14%) or error (10 cases, 5%) rate is much higher than the 10.8% reported by El Helali et al. [27] or the 8.2% claimed by the manufacturer [29]. After retesting according to the manufacturer's instructions, 2 cases were confirmed as positive and the remaining 32 cases were confirmed as negative. The major causes of invalid and error results were PCR inhibition and high syringe pressure exceeding the cut-off point, respectively. Generally, the presence of significant amounts of mucus or feces in a sample is known to inhibit PCR and block the microfluidic channel in the cartridge [27]. This explanation is supported by the observation that the invalid or error rate in our analytical study, which used cell suspension soaked swabs, was approximately 8%. This finding highlights the need to collect specimens in a manner that reduces excessive mucus and feces to avoid unnecessary retesting by the Xpert GBS assay.
The TAT was much shorter for the Xpert GBS assay than for culture; however, the Xpert GBS assay was performed in batch mode, not in real-time mode. If a specimen was tested in realtime mode, the TAT for the Xpert GBS assay can be decreased to less than 3 hr. In a Korean study, the average delivery time for Korean pregnant women was about 5 hr [30]. In fact, because GBS carriage in pregnant women may be intermittent, a prenatal culture might be a poor predictor of maternal GBS carriage at the time of delivery [11, 27, 31]. As a feasible alternative to intrapartum culture, an intrapartum molecular test could change the current practice of prenatal screening between 35 and 37 weeks of gestation. The complete automation and short TAT of the Xpert GBS assay makes it uniquely suited for intrapartum testing. It is simple to perform as an on-demand test by less trained persons, and the results should be available in time for administration of intrapartum antibiotic prophylaxis [27].
In conclusion, we demonstrated that the RT-PCR-based Xpert GBS assay is a rapid and sensitive tool for prenatal diagnosis of GBS compared to the standard culture method, and it could be especially useful for clinical settings where culture is not feasible.
Acknowledgement
Statistical analysis was supported by the Medical Research Collaborating Center of Seoul National University Hospital.
References
1. Kim KA, Shin SM, Choi JH. A nationwide survey of the causative organisms of neonatal sepsis in Korea. J Korean Pediatr Soc. 2002; 45:55–63.
2. Kim TH, Park SE, Kim KH. A study of group B streptococcal infection in pregnant women by LIM broth media. Korean J Pediatr. 2004; 47:1072–1075.
3. Shim GH, Kim SD, Kim HS, Kim ES, Lee HJ, Lee JA, et al. Trends in epidemiology of neonatal sepsis in a tertiary center in Korea: a 26-year longitudinal analysis, 1980-2005. J Korean Med Sci. 2011; 26:284–289. PMID: 21286023.

4. Choi KU, Koh SK, Lee JY, Park JH, Hwang SO, Lee BI, et al. Clinical significance of group B streptococcal infection in pregnant women. Korean J Obstet Gynecol. 2002; 45:811–815.
5. Jordan JA, Hall G, Davis T. Multicenter study evaluating performance of the Smart Group B Streptococcus (GBS) assay using an enrichment protocol for detecting GBS colonization in patients in the antepartum period. J Clin Microbiol. 2010; 48:3193–3197. PMID: 20668132.

6. Lee SH, Park K, Lee HK, Kim MY, Kim JY, Kwon WK, et al. Perinatal colonization rate and antimicrobial susceptibility of group B streptococcus in pregnant and non-pregnant Korean women. Korean J Clin Microbiol. 2009; 12:180–185.
7. Kim EJ, Oh KY, Kim MY, Seo YS, Shin JH, Song YR, et al. Risk factors for group B streptococcus colonization among pregnant women in Korea. Epidemiol Health. 2011; 33:e2011010. PMID: 22111030.

8. Park LS, Seo K, Kim SK, Park YW, Jung HY, Chong YS, et al. A study of group B streptococcal infection in Korean pregnant women. Korean J Obstet Gynecol. 1999; 42:2038–2042.
9. Yi K, Yong DG, Cho DH, Lee KW, Kim DS, Chong YS. Trend in the isolation of hemolytic streptococci and current infection status of group B streptococcus. Korean J Clin Pathol. 2001; 21:365–370.
10. Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010; 59:1–36. PMID: 21088663.
11. Davies HD, Miller MA, Faro S, Gregson D, Kehl SC, Jordan JA. Multicenter study of a rapid molecular-based assay for the diagnosis of group B streptococcus colonization in pregnant women. Clin Infect Dis. 2004; 39:1129–1135. PMID: 15486835.

12. Yancey MK, Schuchat A, Brown LK, Ventura VL, Markenson GR. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol. 1996; 88:811–815. PMID: 8885919.

13. Block T, Munson E, Culver A, Vaughan K, Hryciuk JE. Comparison of carrot broth- and selective Todd-Hewitt broth-enhanced PCR protocols for real-time detection of Streptococcus agalactiae in prenatal vaginal/anorectal specimens. J Clin Microbiol. 2008; 46:3615–3620. PMID: 18799703.
14. Montague NS, Cleary TJ, Martinez OV, Procop GW. Detection of group B streptococci in Lim broth by use of group B streptococcus peptide nucleic acid fluorescent in situ hybridization and selective and nonselective agars. J Clin Microbiol. 2008; 46:3470–3472. PMID: 18667597.
15. Ke D, Menard C, Picard FJ, Boissinot M, Ouellette M, Roy PH, et al. Development of conventional and real-time PCR assays for the rapid detection of group B streptococci. Clin Chem. 2000; 46:324–331. PMID: 10702518.

16. Burd EM. Validation of laboratory-developed molecular assays for infectious diseases. Clin Microbiol Rev. 2010; 23:550–576. PMID: 20610823.

17. Centers for Disease Control and Prevention. Prevention of perinatal group B streptococcal disease: a public health perspective. MMWR Recomm Rep. 1996; 45:1–24.
18. Philipson EH, Palermino DA, Robinson A. Enhanced antenatal detection of group B streptococcus colonization. Obstet Gynecol. 1995; 85:437–439. PMID: 7862387.

19. Valkenburg-van den Berg AW, Sprij AJ, Oostvogel PM, Mutsaers JA, Renes WB, Rosendaal FR, et al. Prevalence of colonisation with group B streptococci in pregnant women of a multi-ethnic population in The Netherlands. Eur J Obstet Gynecol Reprod Biol. 2006; 124:178–183. PMID: 16026920.
20. Meyn LA, Krohn MA, Hillier SL. Rectal colonization by group B streptococcus as a predictor of vaginal colonization. Am J Obstet Gynecol. 2009; 201:76.e1–76.e7. PMID: 19371857.

21. Hansen SM, Sorensen UB. Method for quantitative detection and presumptive identification of group B streptococci on primary plating. J Clin Microbiol. 2003; 41:1399–1403. PMID: 12682120.

22. Cheng PJ, Chueh HY, Liu CM, Hsu JJ, Hsieh TT, Soong YK. Risk factors for recurrence of group B streptococcus colonization in a subsequent pregnancy. Obstet Gynecol. 2008; 111:704–709. PMID: 18310374.

23. Edwards M, Baker C. Remington J, Klein J, editors. Group B streptococcal infections. Infectious Diseases of the Fetus and Newborn Infant. 2001. Philadelphia: WB Saunders;p. 1091–1156.

24. Smith D, Perry JD, Laine L, Galloway A, Gould FK. Comparison of BD GeneOhm real-time polymerase chain reaction with chromogenic and conventional culture methods for detection of group B streptococcus in clinical samples. Diagn Microbiol Infect Dis. 2008; 61:369–372. PMID: 18440176.

25. Dunne WM Jr, Holland-Staley CA. Comparison of NNA agar culture and selective broth culture for detection of group B streptococcal colonization in women. J Clin Microbiol. 1998; 36:2298–2300. PMID: 9666009.

26. Church DL, Baxter H, Lloyd T, Miller B, Gregson DB. Evaluation of the Xpert(R) group B streptococcus real-time polymerase chain reaction assay compared to StrepB Carrot Broth for the rapid intrapartum detection of group B streptococcus colonization. Diagn Microbiol Infect Dis. 2011; 69:460–462. PMID: 21396547.
27. El Helali N, Nguyen JC, Ly A, Giovangrandi Y, Trinquart L. Diagnostic accuracy of a rapid real-time polymerase chain reaction assay for universal intrapartum group B streptococcus screening. Clin Infect Dis. 2009; 49:417–423. PMID: 19580414.

28. Gavino M, Wang E. A comparison of a new rapid real-time polymerase chain reaction system to traditional culture in determining group B streptococcus colonization. Am J Obstet Gynecol. 2007; 197:388.e1–388.e4. PMID: 17904971.

29. Cepheid. Xpert GBS. Updated on Sep 2011. http://www.diagnostictechnology.com.au/persistent/catalogue_files/products/xpertgbspi.pdf.
30. Kwon JY, Lee Y, Suh MJ, Kim SJ, Shin JC, Lee JG, et al. The length of active labor in women with vaginal birth after cesarean section compared with nulliparas and multiparas. Korean J Obstet Gynecol. 2005; 48:2843–2849.
31. Hiller JE, McDonald HM, Darbyshire P, Crowther CA. Antenatal screening for Group B Streptococcus: a diagnostic cohort study. BMC Pregnancy Childbirth. 2005; 5:12. PMID: 16042773.

Table 2
Diagnostic performance of the Xpert GBS assay and the standard culture method for the detection of GBS colonization
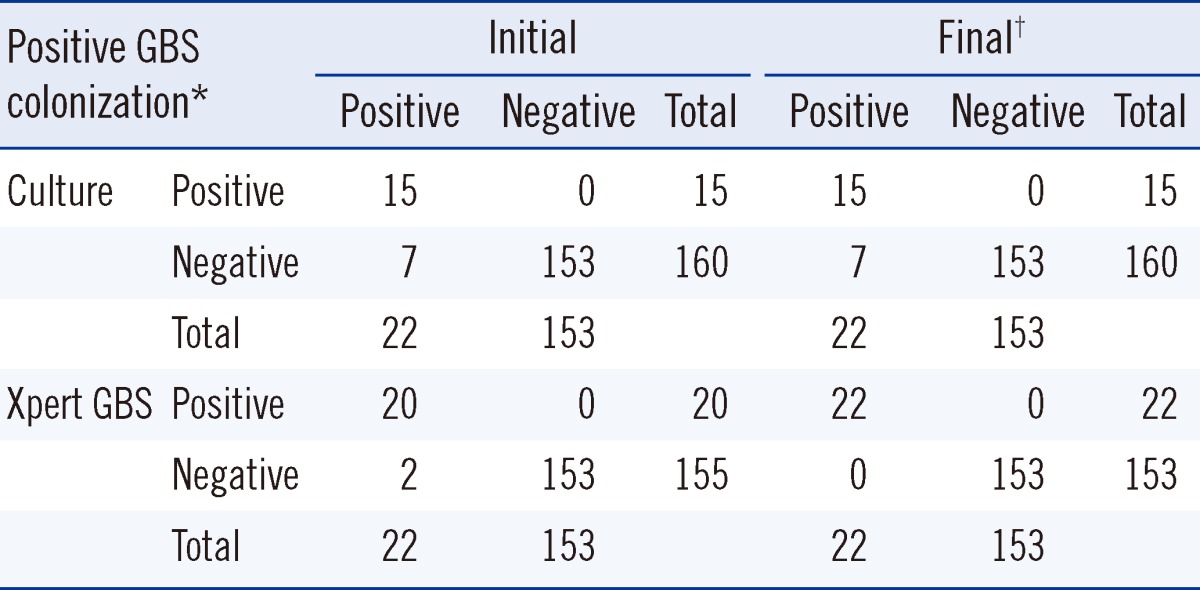
*Positive GBS colonization was defined as a case for which either the Xpert GBS assay, standard culture method, or both yielded positive results.
†Final diagnostic performance was determined after retesting samples from 9 discrepant cases between the Xpert GBS and culture. While 7 cases with negative culture results remained negative upon repeated culture of the archived swabs, 2 cases with negative Xpert GBS results were confirmed to be positive upon repeated testing of stored frozen isolates.
Abbreviation: GBS, group B streptococcus.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download