Klebsiella pneumoniae is a common nosocomial pathogen causing pneumonia and bacteremia in intensive care units (ICUs). Since the introduction of extended-spectrum cephalosporins, extended-spectrum β-lactamase (ESBL)-producing K. pneumoniae has emerged and caused serious problems worldwide. Until recently, ESBL-producing K. pneumoniae was relatively uncommon in Japan, where Japan Nosocomial Infections Surveillance data (http://idsc.nih.go.jp/iasr/index-j.html) from 2001 show that its resistance rates to cefotaxime and ceftazidime were only 1.6% and 1.1%, respectively. ESBL-producing Enterobacteriacea isolates have been reported more frequently during the past few years and are gradually becoming a serious concern.
We recently encountered a case of aspiration pneumonia caused by K. pneumoniae, in which empirical antimicrobial therapy using carbapenem failed. Later, the causative agent was identified as a recently described K. pneumoniae phenotype showing an unusual susceptibility to carbapenems. This phenotype is susceptible to imipenem (IPM) but resistant to other carbapenems (imipenem-susceptible, meropenem-resistant Klebsiella, ISMRK) [1]. To our knowledge, this is the first known report of a fatal case of ISMRK infection, in which IPM was not effective.
A 70-yr-old man with a history of infectious endocarditis and bullous pemphigoid requiring steroid treatment (prednisolone, 20 mg/day) was admitted to the hospital with pharyngeal hemorrhage. On hospital day 5, he developed upper gastrointestinal bleeding and respiratory distress. Although enteral feeding via a nasogastric tube was started on day 10, he developed aspiration pneumonia on day 20; therefore, enteral feeding was stopped, and he was managed with total parenteral nutrition. On hospital day 30, his blood pressure decreased and a computed tomography scan showed consolidation of the dorsal lower lobe of the right lung, suggesting septic shock due to aspiration pneumonia. The patient was transferred to the ICU where mechanical respiratory ventilation was required. On the same day, bacterial culture of venous blood revealed the presence of K. pneumoniae ("K1"), and treatment with ampicillin/sulbactam (1.5 g intravenously [i.v.] every 12 hr) was initiated.
On hospital day 31, blood endotoxins were identified, and the patient was treated with both continuous hemodiafiltration and polymyxin B hemoperfusion. After K1 was found to be ESBL-positive, empirical antimicrobial treatment was changed from ampicillin/sulbactam to meropenem (MEM, 1 g i.v. every 8 hr). Additionally, on hospital day 31, a nasal swab, respiratory secretions obtained by tracheal suction, intravenous hyperalimentation, and arterial blood were bacterially cultured. K. pneumoniae was detected from the nasal swab ("K2"), respiratory secretions ("K3"), and arterial blood ("K4" and "K5"). On hospital day 32, susceptibility testing revealed that these isolates were resistant to MEM, and treatment with amikacin (400 mg i.v. per day) and IPM/cilastatin (0.5 g i.v. every 8 hr) was started. However, the patient's condition did not improve, and he died of respiratory failure on hospital day 33.
Antimicrobial susceptibility tests were performed for all 5 K. pneumoniae isolates collected from this patient, by using the broth micro-dilution method and MicroScan WalkAway system (Siemens Healthcare Diagnostics, Tokyo, Japan). All 5 isolates were highly resistant to almost all β-lactam antibiotics, with the notable exception of IPM, including piperacillin (<16 µg/mL), and were intermediately resistant to amoxicillin/clavulanic acid (16 µg/mL). The minimum inhibitory concentration (MIC) of MEM was >8 µg/mL. In contrast, the MIC of IPM was 1 µg/mL. Given the susceptibility pattern, the isolates were suspected to be ISMRK [1] and were phenotypically and genetically evaluated.
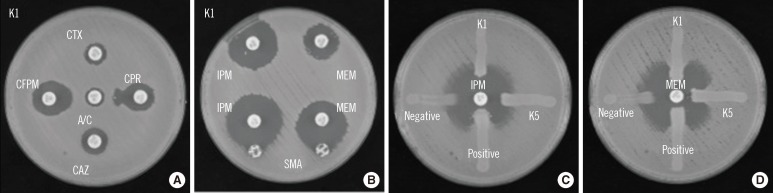
Identification of ESBL and metallo-β-lactamase (MBL) activities was performed using a double-disk synergy test (DDST), a sodium mercaptoacetic acid (SMA) test, and a modified Hodge test (MHT) [2] (Fig. 1). Each of the 5 isolates (K1-K5) was positive for cefepime (FEP) and cefpirome (CPR) by DDST, suggesting the production of ESBL. Additionally, on MHT, all 5 isolates were found to be positive for MEM but negative for IPM. Conversely, all 5 isolates were positive by SMA. The presence of genes in the IMP-1 and CTX-M-2 families was confirmed by PCR mapping of the integron [1]. Furthermore, nucleotide sequencing of the PCR products for blaIMP-1-type isolates showed sequences identical to blaIMP-6 (DDBJ AB616660). Multi-locus sequence typing of K1 indicated that the isolate belonged to ST37, the same sequence type of ISMRK that we previously reported [1]. Pulsed-field gel electrophoresis of the 5 isolates showed indistinguishable genomic DNA patterns, suggesting that the isolates are genetically identical (data not shown). Plasmid profiling and Southern hybridization analyses showed the isolates possessed blaIMP-6 and blaCTX-M-2 on a 50-kb plasmid, as previously reported [1].
ISMRK is a newly characterized K. pneumoniae strain showing an unusual susceptibility profile to carbapenems [1]. ISMRK carries blaIMP-6 and blaCTX-M-2 genes, which are resistant to most β-lactams, except IPM. After the revision of carbapenem breakpoints for Enterobacteriaceae issued by CLSI in June 2010, ISMRK was confirmed to be susceptible to IPM by the micro-dilution method. IMP-6 (a variant of IMP-1 [Ser196Gly]) was first reported in Serratia marcescens in northern Japan, and the kcat/Km value of IMP-6 was significantly lower than IMP-1 for penicillin G and piperacillin/clavulanic acid [3]. K. pneumoniae carrying IMP-6 was first discovered in 2011 in Japan [1]. Since then, dissemination of an IMP-6-producing Pseudomonas aeruginosa has also been reported in Korea [4]. To our knowledge, this is the first report of a fatality caused by ISMRK.
Our medical bacteriology laboratory routinely uses a susceptibility panel that includes IPM and MEM for Enterobacteriaceae spp. Therefore, our laboratory was able to identify carbapenem resistance and identify the possibility that these isolates were capable of MBL production. However, medical bacteriology laboratories using susceptibility panels with only IPM as a representative of carbapenems may not identify the carbapenemase production of ISMRK and are likely to report the isolate as an ESBL-producing Klebsiella sp. In the case reported here, the patient's treatment was changed from MEM to IPM/cilastatin after susceptibility test results were reported on day 32, but the patient did not recover. Previous studies indicated that standard susceptibility tests often categorize MBL-producing Enterobacteriaceae spp. as susceptible to carbapenems [5, 6]; however, clinical data using carbapenem treatment for carbapenem-susceptible Enterobacteriaceae spp. producing MBL is limited. Galani et al. [5] reported that IPM therapy failed to treat a catheter-related bacteremia caused by an IPM-susceptible Enterobacter cloacae carrying blaVIM-1. Additionally, Mochon et al. [7] reported that empirical MEM therapy was administered to a patient with K. pneumoniae carrying blaNDM-1, and the strain was susceptible to ertapenem, IPM, and MEM; however, this treatment was discontinued because of the increase in the MICs of the isolates to those drugs used during the therapy. These cases show a lack of correlation between in vitro results and treatment efficacy. In vivo experiments using animal models may be warranted to evaluate the efficacy of IPM for the treatment of ISMRK infections.
In summary, we report the first known case of septicemia caused by ISMRK. The emergence of ISMRK suggests the need for a consensus guideline to interpret the anti-biograms of Enterobacteriaceae spp.
References
1. Shigemoto N, Kuwahara R, Kayama S, Shimizu W, Onodera M, Yokozaki M, et al. Emergence in Japan of an imipenem-susceptible, meropenem-resistant Klebsiella pneumoniae carrying blaIMP-6. Diagn Microbiol Infect Dis. 2012; 72:109–112. PMID: 22078906.
2. Clinical and Laboratory Standars Institute. Performance standards for antimicrobial susceptibility testing. 20th Informational supplement. M100-S20. Wayne, PA: CLSI;2010.
3. Yano H, Kuga A, Okamoto R, Kitasato H, Kobayashi T, Inoue M. Plasmid-encoded metallo-beta-lactamase (IMP-6) conferring resistance to carbapenems, especially meropenem. Antimicrob Agents Chemother. 2001; 45:1343–1348. PMID: 11302793.
4. Yoo JS, Yang JW, Kim HM, Byeon J, Kim HS, Yoo JI, et al. Dissemination of genetically related IMP-6-producing multidrug-resistant Pseudomonas aeruginosa ST235 in South Korea. Int J Antimicrob Agents. 2012; 39:300–304. PMID: 22325122.
5. Galani I, Souli M, Chryssouli Z, Orlandou K, Giamarellou H. Characterization of a new integron containing blaVIM-1 and aac(6')-IIc in an Enterbacter cloacae clinical isolate from Greece. J Antimicrob Chemother. 2005; 55:634–638. PMID: 15761066.
6. Giakkoupi P, Tzouvelekis LS, Daikos GL, Miriagou V, Petrikkos G, Legakis NJ, et al. Discrepancies and interpretation problems in susceptibility testing of VIM-1-producing Klebsiella pneumoniae isolates. J Clin Microbiol. 2005; 43:494–496. PMID: 15635025.
7. Mochon AB, Garner OB, Hindler JA, Krogstad P, Ward KW, Lewinski MA, et al. New Delhi metallo-β-lactamase (NDM-1)-producing Klebsiella pneumoniae: case report and laboratory detection strategies. J Clin Microbiol. 2011; 49:1667–1670. PMID: 21325558.
Fig. 1
Phenotypic detection of extended spectrum β-lactamase and carbapenemases in the K1 isolate, an imipenem-susceptible meropenem-resistant Klebsiella pneumoniae strain isolated from venous blood. (A) DDST with amoxicillin/clavulanate. K. pneumoniae K1 was inoculated onto the surface of a Muller-Hinton agar plate. Cephalosporin disks were applied at a distance of 20 mm from the clavulanate-containing disk; an enhanced zone of inhibition toward the clavulanate-containing disk was observed. (B) SMA test using IPM and MEM. K. pneumoniae K1 was inoculated onto the surface of a Muller-Hinton agar plate. An SMA disk was placed adjacent to either the IPM or MEM disk; an enhanced zone of inhibition toward the SMA disk was observed. (C) Modified Hodge test using IPM or MEM. E. coli ATCC25922 was inoculated onto the surface of a Muller-Hinton agar plate and organisms (K1, K5, K. pneumoniae ATCC BAA-1705 for positive control and K. pneumoniae ATCC BAA-1706 for negative control) were inoculated as streaks.
Abbreviations: A/C, amoxicillin/clavulanate; CTX, cefotaxime; CPR, cefpirome; CAZ, ceftazidime; CFPM, cefepime; DDST, double-disk synergy test; IPM, imipenem; MEM, meropenem; SMA, sodium mercaptoacetic acid.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download