Abstract
Background
The aim of this study was to investigate the frequency of autoantibodies with mimicking specificity by using the dilution technique, to assess the usefulness of the combination of the dilution technique and red blood cell (RBC) phenotyping, and to establish a pre-transfusion testing algorithm in patients with warm autoantibodies.
Methods
Serum samples from 71 patients with warm autoantibodies were tested using the dilution technique. Among them, 25 samples were adsorbed with allogeneic ZZAP (a combination of dithiothreitol and enzyme) or polyethylene glycol (PEG) and their RBC phenotypes were determined. Thirty-nine patients were transfused with our pre-transfusion testing algorithm using a combination of dilution technique and RBC phenotyping.
Results
Autoantibodies with mimicking specificity were detected by the dilution technique in 26.8% (19/71) of the patients and most of them were directed against Rh system antigens. The agreement of the results obtained with the dilution technique in combination with RBC phenotyping and those from ZZAP or PEG adsorption was 100% (18/18) in patients who have autoantibodies with mimicking specificity and/or alloantibodies. No clinical symptoms indicating severe acute or delayed hemolytic transfusion reactions were reported in the 39 patients transfused with our pre-transfusion testing algorithm.
Conclusions
Autoantibodies with mimicking specificity detected by the dilution technique in patients with warm autoantibodies are relatively frequent, can be discriminated from alloantibodies by employing a combination of dilution technique and RBC phenotyping, and might not appear to cause severe acute or delayed hemolytic transfusion reactions.
In the pre-transfusion testing of patients whose sera contain warm autoantibodies, it must be determined whether alloantibodies, which could be masked by the autoantibodies, are also present [1]. To date, application of the dilution technique on patients' sera and adsorption techniques employing polyethylene glycol (PEG) and ZZAP (a combination of dithiothreitol and enzyme) have been developed [2-4]. Among them, it has been reported that the most reliable methods are the adsorption procedures using allogeneic or autologous red blood cells (RBCs) treated with enzymes or ZZAP reagent [3, 5]. However, these methods are time consuming [3].
In contrast to the ZZAP method, the dilution technique is a simple procedure capable of detecting alloantibodies masked by autoantibodies [2]. Experimentally, it consists of testing diluted serum sample against a panel of RBC reagents. Although dilution should be performed only when the alloantibody titer is higher than the autoantibody titer, the dilution technique has been used in some laboratories that do not have the capability to perform the adsorption methods (e.g., ZZAP and PEG) [1].
In our hospital laboratory, the dilution technique has been performed since 2009. In contrast to a previous report [6], a high frequency of autoantibodies with mimicking blood group specificity was detected with this technique.
Therefore, we investigated the frequency of the autoantibodies with mimicking specificity by using the dilution technique on serum samples from patients with warm autoantibodies, compared the dilution technique with ZZAP or PEG adsorption methods, and established the RBC transfusion workflow by using a combination of dilution technique and RBC phenotyping.
Serum samples were selected among 54,848 patients who underwent pre-transfusion investigations at the Chonnam National University Hospital (Gwangju, Korea) and Chonnam National University Hwasun Hospital (Jeollanam-do, Korea) between November 2009 and November 2011.
Serologic techniques were performed according to the American Association of Blood Banks (AABB) standards [7]. We determined the RBC phenotype of the patients at least three months after the last transfusion by using the commercially available phenotyping gel card RhD+Phenotype (DiaMed GmBH, Cressier, Switzerland). In some patients, Rh phenotypes were confirmed by determining the RBC phenotype after ZZAP treatment or by using RHD and RHCE genotyping [8].
A warm autoantibody was defined as an antibody that reacts at 37℃ with antihuman globulin (AHG) and with the patient's own RBCs. In this study, the detection was carried out with the entire panel of 11 RBC reagents by using the ID-DiaPanel system (DiaMed GmBH). To confirm the presence of an autoantibody, a direct antiglobulin test (DAT) was also performed when patients with suspected autoantibodies were identified. The DAT was performed using the DC-Screening I gel card (DiaMed GmBH). When a warm autoantibody was confirmed according to the above-described criteria, we performed the dilution technique to reduce the titer of the autoantibodies, thereby allowing the detection of any underlying alloantibodies.
We performed the dilution technique as previously described [9]. Briefly, we diluted the serum samples in saline by performing 2-fold serial dilutions until a 1+ reaction was achieved in the column agglutination method using LISS/Coombs card (DiaMed GmBH) and ID-DiaCell I+II (DiaMed GmBH). Then, we performed alloantibody identification by mixing the diluted serum with the 11 RBC reagents on the panel by using the ID-DiaPanel system.
Alloadsorption was performed as previously described to discriminate between alloantibodies and autoantibodies with mimicking specificity [1]. Autoantibodies with mimicking specificity were considered antibodies that display apparent antigen specificity and that do not maintain specificity following alloadsorption [6].
From November 2009 to November 2011, pre-transfusion investigations were performed on 54,848 patients, of which 553 underwent antibody identification testing by the gel column agglutination method. Seventy-five patients with warm autoantibodies were detected and the dilution technique combined with RBC phenotyping was applied to the serum samples of 71 of those patients to find alloantibodies and/or autoantibodies with mimicking specificity. Among those 71 patients, by using the combination of dilution technique and red cell phenotyping to discriminate autoantibodies with mimicking specificity from possible alloantibodies, we found that 26.8% had autoantibodies with mimicking specificity. Autoanti-C+e, autoanti-e, autoanti-E, autoanti-C, and autoanti-D were the most common mimicking specificities in the order of detection rate. The specificities of the antibodies in those 71 patients are listed in Table 1.
Of the 71 samples, 25 provided enough volume for further serologic evaluation using ZZAP or PEG adsorptions to discriminate the autoantibodies with mimicking specificity from the alloantibodies. The dilution fold of the samples that resulted in a 1+ reaction in the column agglutination method using the LISS/Coombs card varied from 1:2 to 1:512. In patients who have autoantibodies with mimicking specificity and/or alloantibodies, the agreement of the results obtained with the dilution technique in combination with RBC phenotyping and those from ZZAP or PEG adsorption was 100% (18/18). We also found that all autoantibodies with mimicking specificity detected in our study were completely removed by ZZAP or PEG alloadsorption. In patients with warm autoantibodies without mimicking specificity detected by the dilution technique, the adsorption methods did not identify any alloantibody except in case 4, for which the warm autoantibodies might not have been completely adsorbed by ZZAP (Table 2).
In our retrospective investigation, we transfused "least incompatible" RBCs to 17 patients who shows unidentified pattern in antibody identification test, 10 patients with warm autoantibodies without mimicking specificity, and 5 patients with autoantibodies with mimicking specificity; moreover, we transfused antigen-negative blood to 7 patients with possible alloantibodies identified with our algorithm using a combination of dilution technique and RBC phenotyping (Fig. 1). No clinical symptoms indicating severe acute or delayed hemolytic transfusion reactions were reported in the 39 patients who were transfused with the pre-transfusion testing algorithm.
The dilution technique was first proposed by Petz and Garratty as an alternative method for identifying the underlying alloantibodies masked by warm autoantibodies [9]. Although the ZZAP adsorption method is recognized as the most reliable method to eliminate autoantibodies, the dilution technique has been employed for urgent RBC transfusion in some hospital laboratories that do not have the capability to perform ZZAP and/or PEG adsorptions [1].
In our hospital laboratory, the dilution technique has been performed since 2009. To the best of our knowledge, this is the first study to indicate that the detection of autoantibodies with mimicking specificity is relatively common, and to propose a simple screening method for the discrimination of autoantibodies with mimicking specificity from alloantibodies in patients with warm autoantibodies.
There are more patients with warm autoantibodies having mimicking specificity than patients with both warm autoantibodies and alloantibodies. Furthermore, in our study, most of the autoantibodies with mimicking specificity proved to be directed against the Rh system antigens, such as C+e, e, C, and D, thereby making it very difficult to find compatible RBC units for the Korean people. Although they display apparent antigen specificity, our results suggest that many of the antibodies identified with the dilution technique were not true alloantibodies and the mimicking autoantibodies are commonly present in patients with warm autoantibodies.
Mimicking autoantibodies were first described by Funderberg et al. [10] as autoantibodies having the "wrong" specificity, and are generally considered "not common" in routine blood bank serology [6]. To date, very few studies reporting the frequency and specificity of the autoantibodies with mimicking specificity were performed. Issitt et al. [11] reported that for 21.0% (29/138) of the patients with warm autoantibodies, partially adsorbed autoantibodies appeared mimicked alloantibodies in ZZAP adsorption method. Wheeler et al. [12] used alloadsorption and found that 12.0% (12/100) of the serum sample showing positive DAT contained mimicking autoantibodies. The frequencies reported in the previous reports are much lower than the frequency (26.8%) reported in this study. In addition, in our experiments, all the autoantibodies with mimicking specificity revealed by the dilution technique were completely removed by ZZAP or PEG alloadsorption. We suggest that the dilution technique makes the autoantibodies with mimicking specificities more detectable than do ZZAP or PEG adsorption, by producing non-specific partial adsorption of the warm autoantibodies.
It was proposed that since warm antibodies are continually adsorbed by the patient RBCs, it is likely that the amount of autoantibodies left in the serum is less than that of alloantibodies. Therefore, if the patient serum is diluted, the more concentrated alloantibodies become more obvious [13]. Øyen et al. [2] reported that no clinically significant alloantibodies were missed by the serum dilution method compared to allogeneic adsorptions by using the 1:5 dilution protocol. In contrast, Leger et al. [1] reported that 10 potentially clinically significant alloantibodies were not identified because they were masked by the autoantibodies even after diluting the sample 1:5. Since in our method, the dilution fold used for detecting antibody specificities was variable, it might be possible that antibody specificities in the previous studies were masked by warm autoantibodies or eliminated in the 1:5 diluted samples.
Although the warm autoantibodies were not completely removed by dilution, the combination with RBC phenotyping helped the identification and discrimination between autoantibodies with mimicking specificity and alloantibodies. A simple algorithm that combines the dilution technique and RBC phenotyping was introduced in our antibody identification protocol to treat patients with warm autoantibodies (Fig. 1).
When RBC transfusion is necessary for the patients with autoantibodies with mimicking specificity, the use of antigen-negative RBCs is generally considered [6]. To date, no studies on RBC transfusion to patients with autoantibodies with mimicking specificity have been performed, except for a few cases. Yun et al. [14] reported a case of one unit of e-positive packed RBCs (PRCs) that was transfused to a patient who had an autoanti-e mimicking autoantibody without inducing acute hemolytic transfusion reaction or other severe transfusion reactions. Issitt et al. [11] speculated that if the patient serum contains a mimicking autoantibody, it might not be necessary to provide antigen-negative RBCs for transfusion. In the present study, we transfused "least incompatible" RBCs to five patients having autoantibodies with apparent mimicking specificity (Fig. 1), but we did not observe any clinical symptoms indicating severe acute or delayed hemolytic transfusion reactions. Furthermore, although we discovered a high frequency of autoantibodies with mimicking specificity in the sera of patients with warm autoantibodies, neither severe acute nor delayed hemolytic transfusion reactions have been reported after numerous "least incompatible" RBC transfusions. Thus, we suggest that autoantibodies with mimicking specificity might not cause acute or delayed hemolytic transfusion reactions.
Therefore, by using the dilution technique as screening test in the patients with warm autoantibodies, the transfusion of antigen-negative RBCs might not be mandatory when the patients have only autoantibodies with mimicking specificity; however, antigen-negative RBCs should be transfused to patients who possibly have alloantibodies.
Acknowledgements
The authors thank Jeong-Gyoo Kim, M.T., Young-Na Park, M.T., and Kyeong-Suk Jeong, M.T., for their technical assistance.
References
1. Leger RM, Garratty G. Evaluation of methods for detecting alloantibodies underlying warm autoantibodies. Transfusion. 1999; 39:11–16. PMID: 9920161.

2. Øyen R, Angeles ML. A simple screening method to evaluate the presence of alloantibodies with concomitant warm autoantibodies. Immunohematology. 1995; 11:85–87. PMID: 15447065.

3. Cid J, Ortín X, Pinacho A, Parra R, Contreras E, Elies E. Use of polyethylene glycol for performing autologous adsorptions. Transfusion. 2005; 45:694–697. PMID: 15847656.

4. Branch DR, Petz LD. A new reagent (ZZAP) having multiple applications in immunohematology. Am J Clin Pathol. 1982; 78:161–167. PMID: 6808844.

5. Branch DR, Petz LD. Detecting alloantibodies in patients with autoantibodies. Transfusion. 1999; 39:6–10. PMID: 9920160.
6. Dwyre DM, Clapper A, Heintz M, Elbert C, Strauss RG. A red blood cell autoantibody with mimicking anti-E specificity. Transfusion. 2004; 44:1287–1292. PMID: 15318850.

7. In : Carson TH, editor. Pretransfusion testing of patient blood. Standards for blood banks and transfusion services. 27th ed. Bethesda: American Association of Blood Banks;2011. p. 33–35.
8. Kim KH, Kim KE, Woo KS, Han JY, Kim JM, Park KU. Primary anti-D immunization by DEL red blood cells. Korean J Lab Med. 2009; 29:361–365. PMID: 19726900.

9. Petz LD, Garratty G, editors. Immune hemolytic anemias. 2nd ed. Piladelphia: Churchill Living stone;2004. p. 375–400.
10. Fundenberg HH, Rosenfield RE, Wasserman LR. Unusual specificity of auto-antibody in auto-immune hemolytic diseases. J Mt Sinai Hosp N Y. 1958; 25:324–329. PMID: 13564182.
11. Issitt PD, Combs MR, Bumgarner DJ, Allen J, Kirkland A, Melroy-Carawan H. Studies of antibodies in the sera of patients who have made red cell autoantibodies. Transfusion. 1996; 36:481–486. PMID: 8669076.

12. Wheeler CA, Calhoun L, Blackall DP. Warm reactive autoantibodies: clinical and serologic correlations. Am J Clin Pathol. 2004; 122:680–685. PMID: 15491963.
13. Garratty G. What do you do when all units are incompatible? Rev Med Inst Mex Seguro Soc. 2005; 43(S1):107–111.
14. Yun HK, Cho D, Chae MJ, Jeon MJ, Suh SP, Ryang DW. No hemolytic transfusion reactions in a patient with the apparent anti-e autoantibody following transfusion of packed red cells with CcDEe phenotype. Korean J Blood Transfus. 2007; 18:116–120.
Fig. 1
Red cell transfusion workflow in 71 patients having warm autoantibodies evaluated with the combination of dilution technique and red cell phenotyping.
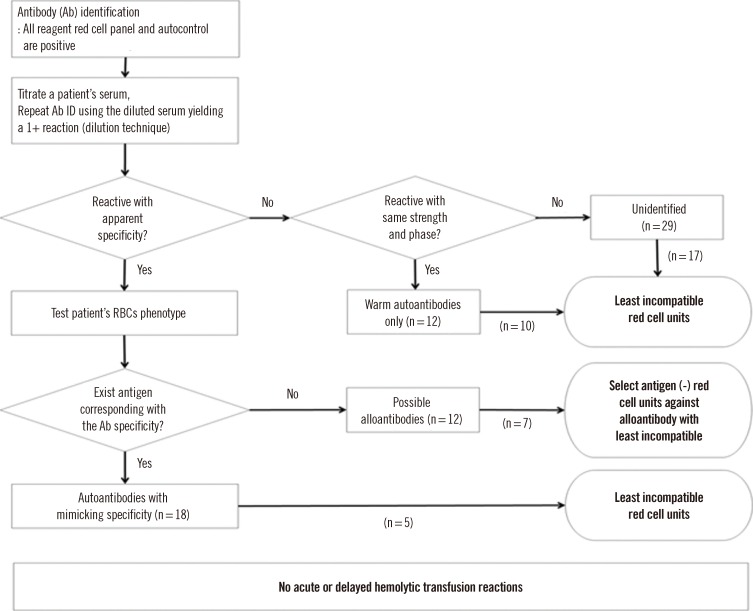
Table 1
Antibody specificities of 71 patient serum samples containing warm autoantibodies as identified by the dilution technique
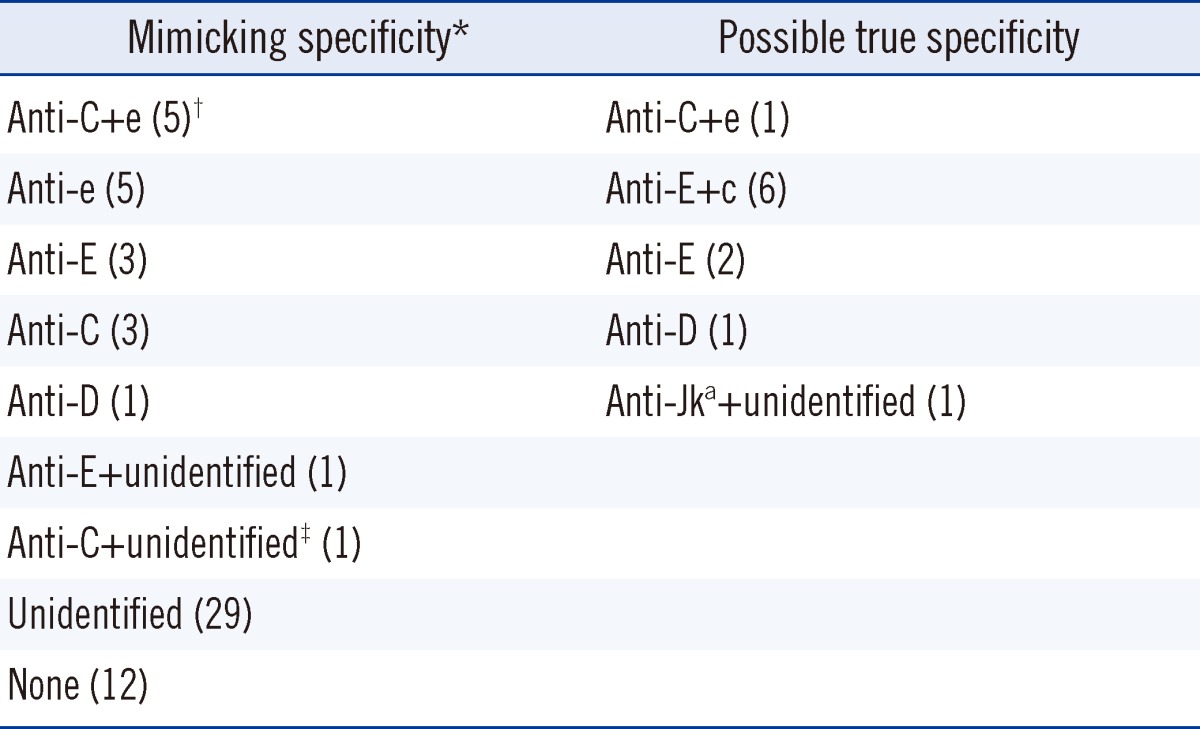
Table 2
Comparison of antibody specificities identified by the dilution technique and adsorption in 25 patients with warm autoantibodies
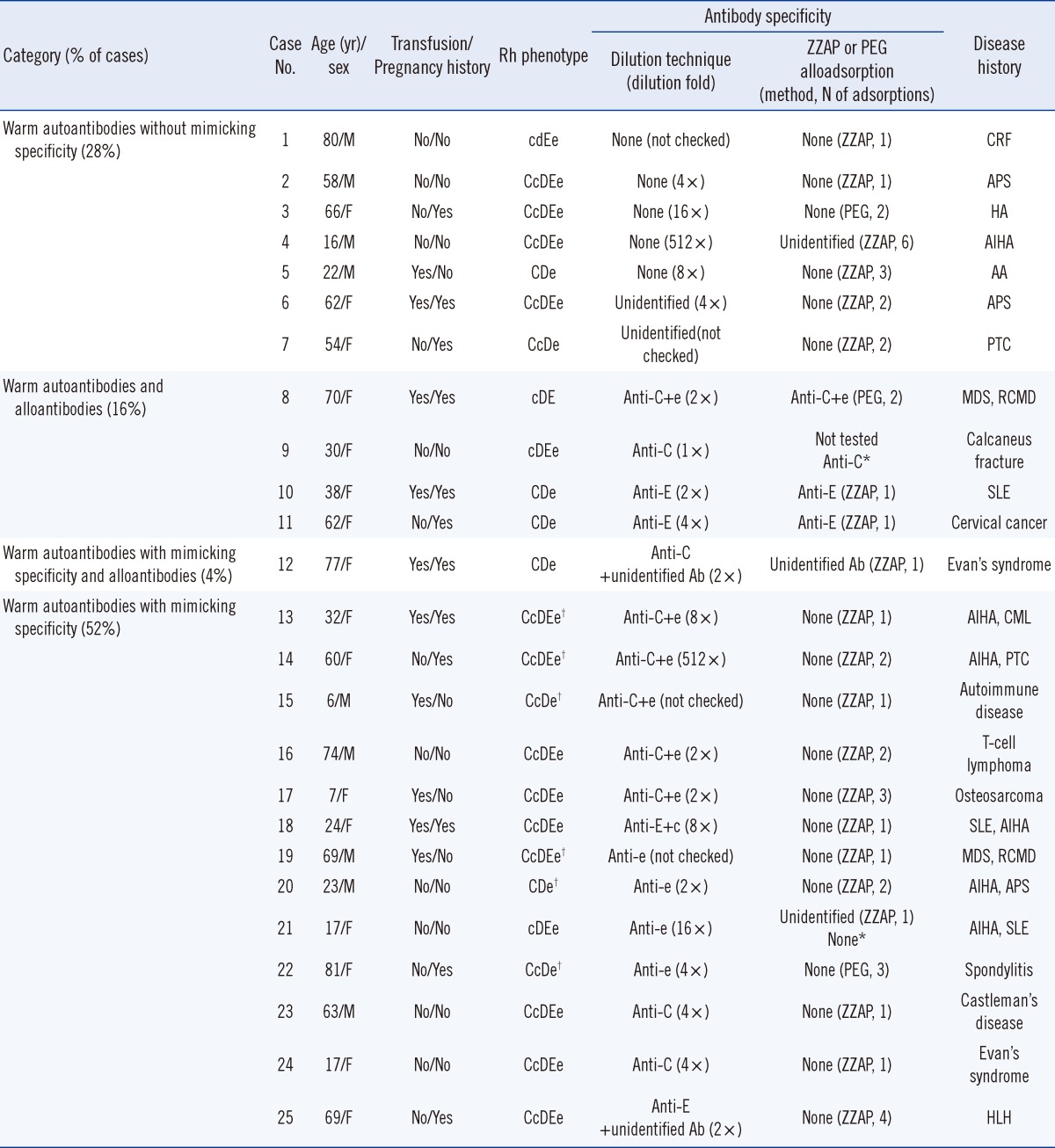
*Antibody specificity was confirmed about six months later without adsorption procedures; †Rh phenotype was confirmed by RHCE genotyping.
Abbreviations: AA, aplastic anemia; AIHA, autoimmune hemolytic anemia; APS, antiphospholipid syndrome; CRF, chronic renal failure; HA, hemolytic anemia; MDS, myelodysplastic syndrome; RCMD, refractory cytopenia with multilineage dysplasia; HLH, hemophagocytic lymphohistiocytosis; PTC, papillary thyroid cancer; SLE, systemic lupus erythematosus.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download