Abstract
Background
D-dimer is used widely as a diagnostic aid in low- and moderate-risk patients with suspected venous thromboembolism (VTE). While our laboratory utilizes VIDAS D-dimer analyzer (bioMérieux SA, France), our emergency department (ED) recently procured a D-dimer analyzer AQT90 FLEX (Radiometer Medical ApS, Denmark) for point of care testing (POCT) to facilitate patient management. We aimed to determine whether the time taken to receive D-dimer results using the 2 different analyzers differed significantly and to quantify the limits of agreement between the results of the 2 methods measured on the same patient.
Methods
Adult patients presenting to the ED and requiring diagnostic workup for suspected VTE were included in this prospective observational study. Patients underwent simultaneous D-dimer measurements using the 2 different analyzers.
Results
The paired results from 104 patients were analyzed. The median time for the D-dimer results from triage by VIDAS was 258 min (Inter-quartile range [IQR], 173-360) and by POCT was 146 min (IQR, 55-280.5); the median time difference was 101.5 min (IQR, 82-125.5). On an average, POCT D-dimer values were 15% lower on the same sample (limits of agreement, 34-213%). POCT predicted 83% of VIDAS positive results (sensitivity, 83.3% [95% confidence interval (CI), 70.4-91.3%]; specificity, 100% [95% CI, 93.6-100%]). All patients with positive imaging were identified correctly by both methods.
D-dimer is widely used as a diagnostic tool in low- and moderate-risk patients with suspected pulmonary embolism (PE) or deep venous thrombosis. In patients with low to moderate pre-test probability, a negative D-dimer result can prevent unnecessary exposure to radiation and contrast and decrease the length of stay in the emergency department (ED), reducing the patient's financial burden [1].
D-dimer is a fibrin degradation product formed by the breakdown of fibrin in vivo by specific enzymes. It is a marker of in vivo fibrin formation, and its measurements may reflect a disturbance in the hemostatic balance. D-dimer levels increase 1 hr following thrombus formation and have a half-life of approximately 8 hr. The presence of D-dimer can be used as an early, sensitive marker for thromboembolic disorders. However, due to its low specificity and high sensitivity, D-dimer can only be used as a screening test in patients with low to moderate risk of a thromboembolic disorder [2]. ELISA methods have increased the sensitivity for detecting elevated D-dimer levels [3, 4]. An added value of fluorescence end point detection methods is that they are fast and have a wide linear range capable of detecting D-dimer levels between 0 and 1,000 µg/mL [5]. Tests using immunofiltration have also decreased the time required for laboratory analysis as compared to that of previous methods [6]. Different methods for detecting D-dimer as a part of the diagnostic work-up for venous thromboembolism (VTE) have been widely reviewed and published in the literature [7]. Furthermore, the diagnostic utility of the VIDAS D-dimer assay in excluding a diagnosis of PE has been validated previously [8].
Our hospital laboratory utilizes the VIDAS D-dimer assay (bioMérieux SA, RCS Lyon, France), which is an automated quantitative test using an enzyme linked fluorescence assay technique. Recently, our ED procured the AQT90 FLEX point of care D-dimer analyzer (Radiometer Medical ApS, Åkandevej, Denmark). AQT90 FLEX is a completely automated continuous access analyzer that utilizes immunoassay technology and time resolved fluorometric detection. The use of AQT90 FLEX D-dimer analyzer has been validated in a prospective trial [9].
Our study had 2 primary objectives. First, we sought to determine whether the time taken to obtain the D-dimer results using laboratory based VIDAS differs significantly from that using the point of care test (POCT). Second, we attempted to quantify the limits of agreement between D-dimer results measured on the same patient using both the D-dimer techniques. A secondary objective was to calculate the sensitivity and specificity of VIDAS and POCT in a subset of patients for whom definitive scan results were available.
Approval was obtained from the western Sydney area health ethics committee. A prospective observational study was conducted at the Adult ED of Westmead Hospital, a tertiary hospital with an annual census of 58,000 patients. The study was conducted from August through December 2010 on patients aged 15 yr or more.
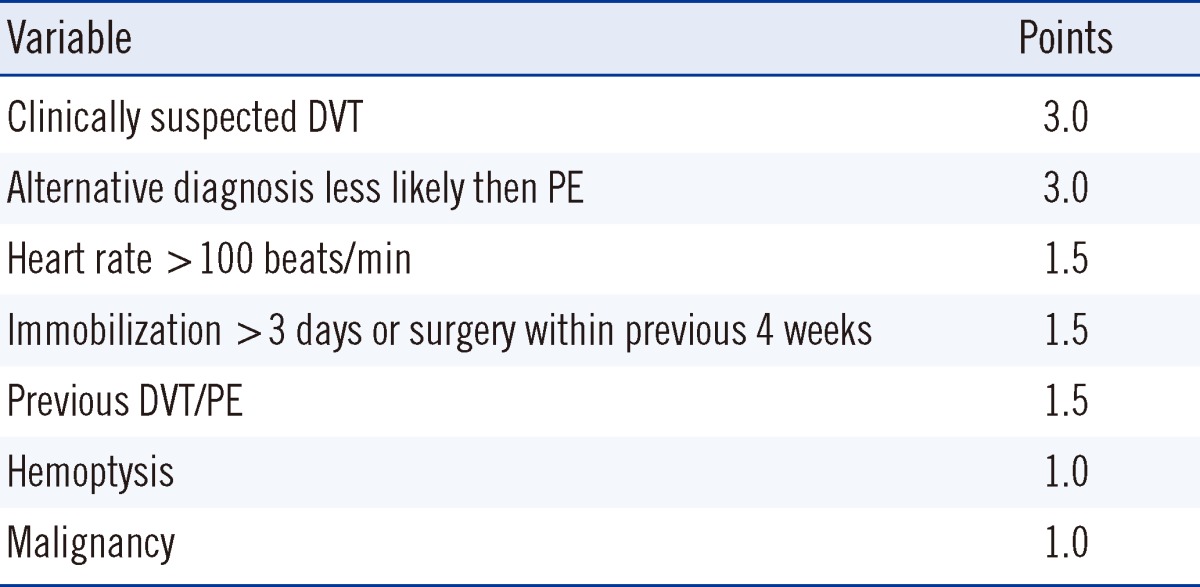
Wells criteria (Table 1) were used by senior medical staff to determine the pre-test probability for PE [10]. High-risk patients were excluded from D-dimer testing and thus, were excluded from the study. Medical staff was trained to use the point of care AQT90 FLEX machine located in the ED. Blood was collected from a single collection site from each patient in a citrated tube and EDTA tube. The citrated tube was sent for laboratory VIDAS D-dimer testing immediately through the pneumatic tube transfer system to the laboratory, whilst the D-dimer analysis on the EDTA tube was performed by medical staff using the AQT90 FLEX point of care analyzer. Medical staff was advised to wait for formal laboratory results prior to making clinical decisions regarding imaging, anticoagulation, and disposition as a part of the validation process. All ongoing management decisions were at the discretion of the treating doctor in ED.
In a pilot study of 20 patients, the observed SD of the difference between the time taken for the VIDAS and POCT results was 115 min for the same patient. Assuming this value of SD, a sample size of 100 patients was calculated to give 80% power for detecting differences between a null hypothesis mean time difference of 0 min and an alternative mean time difference of 45 min using a paired t-test with a 5% two-sided significance level (nQuery Advisor version 7; Statistical Solutions, Cork, Ireland).
Values of the D-dimer results from VIDAS (positive cut-off value ≥500 ng/mL FEU or 0.25 mg/L DD units) and AQT90 FLEX POCT (positive cut off value ≥ 500 ng/mL FEU or 0.25 mg/L DD units) were compared. Triage times (T0), time for the VIDAS D-dimer results made available from PathNet services (T1), and time for the POCT D-dimer results were recorded from the AQT90 automated analyzer log book (T2). Imaging results for patients undergoing computed tomography pulmonary angiography, ventilation-perfusion scanning, and Doppler venous ultrasonography scan were sought from the electronic medical records of patients.
SPSS Version 17 software (SPSS Inc. Chicago, IL, USA) was used for statistical analysis. Bland-Altman plots [11] and the associated "limits of agreement" were used to examine the extent of agreement between VIDAS and AQT90 POCT D-dimer results for the same patient.
Of 112 patients initially screened, 104 were included in the study. Eight patients were excluded from analysis because only POCT D-dimer results were available. The mean age of the subjects was 50.0 ± 19.1 yr (51.9%, men). Within our study population, 90 patients were determined to have moderate probability, and 14 patients had low probabilities for PE as per the Wells criteria.
Time differences were estimated from the triage time of both the methods. The median time for obtaining D-dimer results by VIDAS from the triage time (T1-T0) was 258 min (inter-quartile range [IQR], 173-360 min). The median time for obtaining the POCT results from the triage time (T2-T0) was 146 min (IQR, 55-280.5 min). Time for obtaining the VIDAS D-dimer results was consistently higher than that for the POCT D-dimer results. The median time difference was 101.5 min (IQR, 82-125.5 min). Wilcoxon signed rank test on time for obtaining the results by both the methods revealed that the time for POCT was significantly lower (104 min; 95% CI, 97.5-110.5 min; P<0.0001).
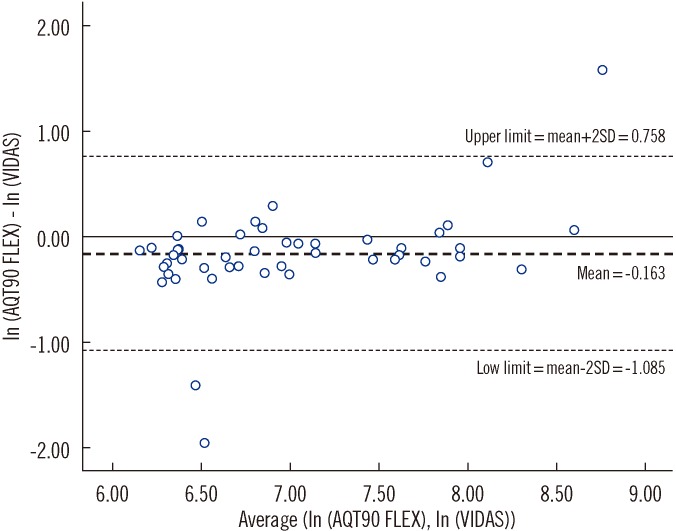
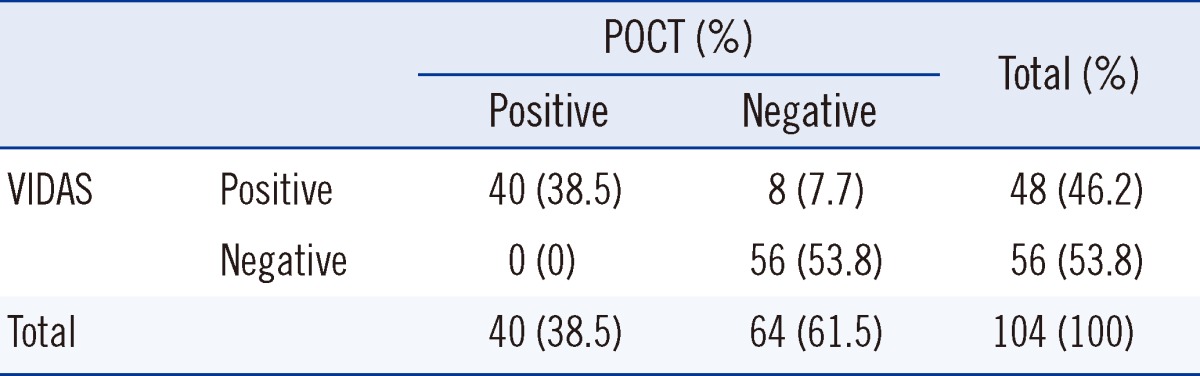
A Bland-Altman plot depicting the VIDAS and POCT D-dimer results is shown in Fig. 1. The size of the within patient differences between the D-dimer results obtained from VIDAS and POCT increased exponentially as the D-dimer levels increased. Thus, log transformation was conducted to check for correlation. On an average, D-dimer values from POCT were 0.85 of those obtained using VIDAS (range, 0.34-2.13). This led to 8 positive results by the VIDAS D-dimer assay being reported as negative by the POCT D-dimer assay (Table 2).
Table 3 displays the D-dimer clinical grouping (positive vs. negative) based on the VIDAS and POCT results. If VIDAS is considered as the gold standard for D-dimer testing, the sensitivity of AQT90 FLEX was 83.3% (95% CI, 70.4-91.3%), and specificity was 100% (95% CI, 93.6-100%).
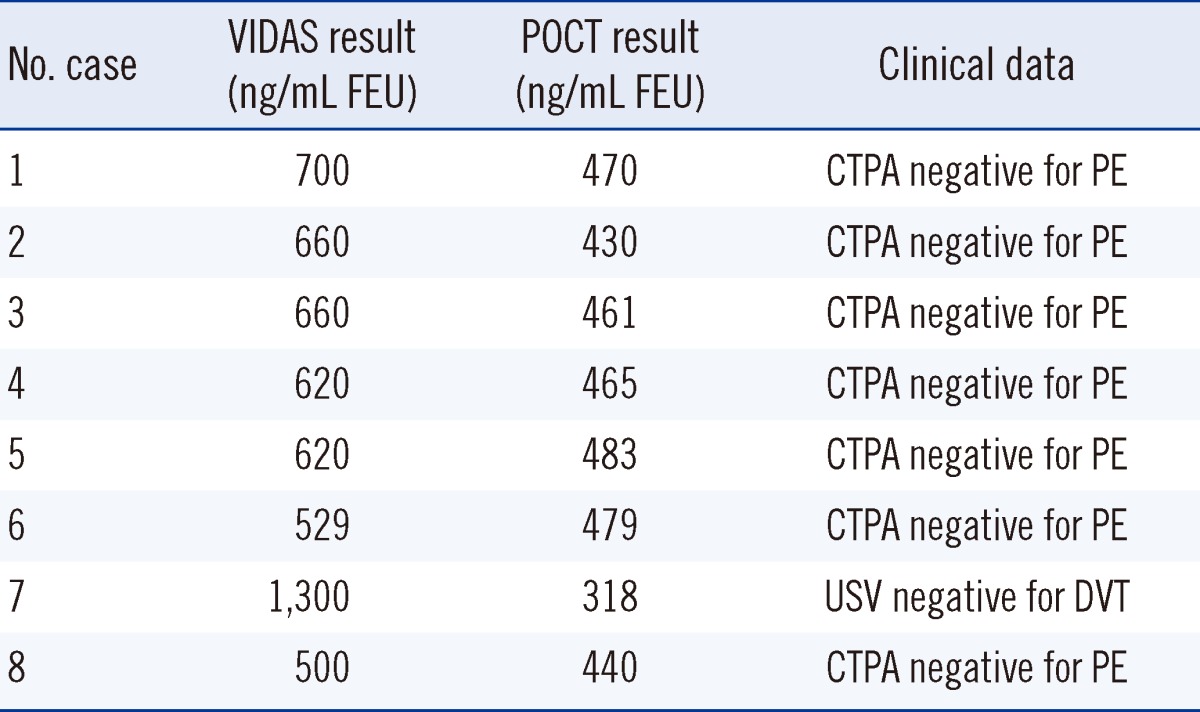
Tables 4 and 5 illustrate the association between imaging results and clinical D-dimer groups based on VIDAS and POCT, respectively. There were 40 patients for whom definitive imaging results were available. Seven patients had positive imaging, and 33 patients had negative imaging results. The negative imaging included computed tomography pulmonary angiography (n=25), ventilation-perfusion scan (n=4) and Doppler venous ultrasonography scan (n=4). All 7 patients with positive imaging were identified correctly by both VIDAS and AQT90 FLEX testing (100% sensitivity). Of the 33 patients with negative imaging results, 6 were identified correctly by VIDAS (specificity, 18%; 95% CI, 8-35%), while 12 were identified correctly by AQT90 FLEX (specificity, 36%; 95% CI, 22-53%). Thus, the AQT90 FLEX had better specificity than VIDAS, resulting in lower rates of false positive results (though still not in the clinically significant range to cause change in current practices). All 8 patients who were reported positive by VIDAS but reported negative by POCT D-dimer underwent imaging. All 8 patients were negative for VTE.
Overcrowding and prolonged length of stay in the ED pose an ongoing threat to patient care in Australia. Lee-Lewandrowski et al. have noted that POCT are becoming increasingly available, and many EDs have adopted them to reduce laboratory turnaround times and expedite clinical decision making [1]. Our results suggest that POCT delivers results with significantly shorter turnaround times than pathology VIDAS services. Our results are similar to those achieved in the past [1].
The Bland-Altman plot demonstrated poor bioequivalence between the VIDAS and AQT90 D-dimer methods. Our study found a significant difference in the D-dimer values between the 2 methods, and higher D-dimer values resulted in increasing differences. This result differs from that achieved by a similar study comparing these methods conducted by Lee-Lewandrowski et al. [1]. This discrepancy could be due to the use of a different analyzer in that study (Biosite triage, Biosite diagnostics, San Diego, CA, USA). The clinical relevance of results variance at higher values of D-dimer would probably be minimal as all results >500 ng/mL FEU would be considered for further intervention.
POCT only detected 83% of the VIDAS positive results. In our study, out of the 48 positive VIDAS results, POCT only yielded 40 positive results. We report a discrepancy of 7.7% (8 of 104) between VIDAS and POCT. This discrepancy raises the issues of the possibility of missing VTE by POCT. However, all 8 patients who had positive VIDAS but negative POCT had negative definitive imaging for VTE. The lower false positive rate for POCT improved its specificity. However, because of the small sample size, the estimated specificity had a wide confidence interval and is still too low to be clinically useful.
In our study, only 15% of VIDAS positive results had positive imaging and approximately 17% of positive POCT had positive imaging, indicating poor specificity of D-dimer testing in diagnosing VTE and leading to unnecessary radiation exposure in some patients. Brotman et al. reported the limitations of D-dimer testing, which is widely recognized in the non-stratified patient [12]. In our study, this was also noted for the low- and moderate-risk stratified patients.
EDs should consider the evidence available for each type of assay being used for POCT. Sidelmann et al. [9]. report that POCT analyzer has negative predictive and positive predictive values as well as specificity and sensitivity that are comparable to routine assays, but these may still result in different D-dimer values, as observed in our study. Despite controversy regarding the diagnostic utility of D-dimer testing in outpatient settings for patients suspected of having venous thromboembolism, POCT of D-dimer can contribute important information and guide patient management when used as part of a diagnostic process as reported by Geersing et al. [13]. The use of D-dimer as a diagnostic tool for VTE is an area of ongoing research. Further studies involving larger sample sizes with clinically suspected VTE are needed to establish the validity of POCT of D-dimer using an imaging technique as a definitive diagnostic test.
Our study has several limitations. Only 6 of the negative D-dimer patients underwent imaging to monitor for VTE. It is possible that the remaining patients could have had a PE or deep venous thrombosis that went undiagnosed. Additionally, it is not known whether patients, who were positive for D-dimer but had negative imaging results, had follow-ups with repeat imaging or a VTE event as this was outside the scope of our study. Our observational study is not immune to selection bias. Though the Wells criteria were followed for sample selection, the decision to order D-dimer remained at the discretion of the evaluating physician. Patients with moderate or low probability for VTE may have proceeded directly to imaging and may not have had a D-dimer testing performed. Finally our sample size was not large enough to conclude the sensitivity, specificity, and predictive values of both the methods in diagnosing or excluding VTE. In conclusion, POCT delivers D-dimer results in significantly shorter turnaround times than pathology services. This could lead to early decision making for patient management with a positive impact on the length of stay at the ED. Poor bioequivalence noted between the VIDAS and POCT suggest that these 2 tests may not be interchangeable and that individual EDs should consider each assay based on its performance measures.
Acknowledgements
Dr. Anna Holgate FACEM, for editorial input; medical and nursing staff of Westmead emergency department; Dr. Karen Byth, Ph.D. biostatistics, for statistical input.
References
1. Lee-Lewandrowski E, Nichols J, Van Cott E, Grisson R, Louissaint A, Benzer T, et al. Implementation of a rapid whole blood D-dimer test in the emergency department of an urban academic medical center: impact on ED length of stay and ancillary test utilization. Am J Clin Pathol. 2009; 132:326–331. PMID: 19687307.
2. Walker JB, Nesheim ME. The molecular weights, mass distribution, chain composition, and structure of soluble fibrin degradation products released from a fibrin clot perfused with plasmin. J Biol Chem. 1999; 274:5201–5212. PMID: 9988770.

3. Greenberg CS, Devine DV, McCrae KM. Measurement of plasma fibrin D-dimer levels with the use of a monoclonal antibody coupled to latex beads. Am J Clin Pathol. 1987; 87:94–100. PMID: 3541576.

4. Dempfle CE. Validation, calibration, and specificity of quantitative D-dimer assays. Semin Vasc Med. 2005; 5:315–320. PMID: 16302152.

5. Pittet JL, de Moerloose P, Reber G, Durand C, Villard C, Piga N, et al. VIDAS D-dimer: fast quantitative ELISA for measuring D-dimer in plasma. Clin Chem. 1996; 42:410–415. PMID: 8598104.

6. Scarano L, Bernardi E, Prandoni P, Sardella C, Rossi L, Carraro P, et al. Accuracy of two newly described D-dimer tests in patients with suspected deep venous thrombosis. Thromb Res. 1997; 86:93–99. PMID: 9175231.

7. Di Nisio M, Squizzato A, Rutjes AW, Buller HR, Zwinderman AH, Bossuyt PM. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemost. 2007; 5:296–304. PMID: 17155963.

8. Perrier A, Roy PM, Aujesky D, Chagnon I, Howarth N, Gourdier AL, et al. Diagnosing pulmonary embolism in outpatients with clinical assessment, D-dimer measurement, venous ultrasound, and helical computed tomography: a multicenter management study. Am J Med. 2004; 116:291–299. PMID: 14984813.

9. Sidelmann JJ, Gram J, Larsen A, Overgaard K, Jespersen J. Analytical and clinical validation of a new point-of-care testing system for determination of D-Dimer in human blood. Thromb Res. 2010; 126:524–530. PMID: 20846709.

10. Wells PS, Hirsh J, Anderson DR, Lensing AW, Foster G, Kearon C, et al. A simple clinical model for the diagnosis of deep-vein thrombosis combined with impedance plethysmography: potential for an improvement in the diagnostic process. J Intern Med. 1998; 243:15–23. PMID: 9487327.

11. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1:307–310. PMID: 2868172.

12. Brotman DJ, Segal JB, Jani JT, Petty BG, Kickler TS. Limitations of D-dimer testing in unselected inpatients with suspected venous thromboembolism. Am J Med. 2003; 114:276–282. PMID: 12681454.

13. Geersing GJ, Janssen KJ, Oudega R, Bax L, Hoes AW, Reitsma JB, et al. Excluding venous thromboembolism using point of care D-dimer tests in outpatients: a diagnostic meta-analysis. BMJ. 2009; 339:b2990. PMID: 19684102.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download