Dear Editor
Hairy cell leukemia (HCL) is a rare lymphoproliferative disorder characterized by an indolent course, progressive pancytopenia, splenomegaly, and infiltration of abnormal B-cells with hairy projections and unique immunophenotypic features [1-3]. Very recently, the acquired V600E mutation of the BRAF gene has been described as a molecular marker for classic HCL (HCLc), and this mutation was not detected in variant HCL (HCLv) and other B-cell neoplasms [4]. However, the degree to which the BRAF V600E mutation can be detected varies according to the detection methods used, types of specimen, or the cell percentages in specimens [5-7]. We describe a case of HCLc with BRAF V600E mutation, which showed discrepant results between bone marrow aspirate and bone marrow biopsy specimens.
A 52-yr-old woman presented with pancytopenia. Her initial blood cell counts were as follows: white blood cells, 1.34×109/L; hemoglobin, 9.9 g/dL; platelets, 23×109/L. Physical examination and computed tomography showed splenomegaly and absence of lymphadenopathy. Her peripheral blood smear showed normocytic normochromic anemia and severe leukopenia characterized by both neutropenia and marked monocytopenia.
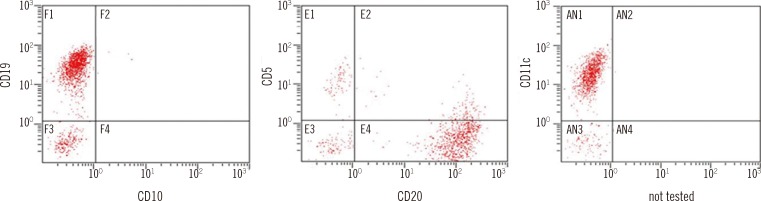
Although it was quite difficult to aspirate the bone marrow, we could confirm the presence of hairy cells on one of the aspirate smears, and abnormal lymphoid cells with inconspicuous nucleoli, frayed cytoplasm, and hairy cytoplasmic projections constituted about 82.7% of all nucleated cells (Fig. 1A). In the bone marrow biopsy section, bone marrow space was packed with abnormal lymphoid cells with abundant cytoplasm, showing a diffuse solid infiltration pattern (Fig. 1B). Reticulin-stained samples showed diffuse and dense coarse bundles of collagen with extensive intersections (Fig. 1C). The cytochemical stain for tartrate-resistant acid phosphatase (TRAP) on touch-print slide could not be interpreted because of poor specimen quality and low percentage of abnormal cells. Immunohistochemically, CD20 (strong) and CD25 were positive (Fig. 1D); CD3 was negative, and kappa and lambda stains showed nonspecific positivity. In flow cytometry analysis, abnormal lymphoid cells were detected in the monocytic region and they were positive for CD11c (strong), CD19, CD20 (strong), HLA-DR, cytoplasmic CD79a, and CD2; and negative for CD3, cytoplasmic CD3, CD5, CD7, CD10, CD13, CD14, CD33, CD34, CD56, and myeloperoxidase (Fig. 2). Chromosome analysis showed a normal karyotype.
The BRAF V600E mutation analysis was performed using a mutation-specific real-time PCR kit (Real Q BRAF V600E detection kit; BioSewoom Inc., Seoul, Korea) and showed discrepant results among the specimens. The BRAF V600E mutation was not detected in the bone marrow aspirates, whereas it was detected in both right and left bone marrow biopsy specimens. The BRAF V600E mutation in the biopsy specimens was confirmed by direct sequencing. The patient was diagnosed as having HCLc and treated with cladribine.
HCLc is a distinct disease characterized by an indolent course and the presence of small mature B lymphoid cells with abundant cytoplasm and hairy projections, involving peripheral blood, bone marrow, and splenic red pulp [8]. The differential diagnosis of HCLc includes chronic lymphocytic leukemia/small lymphocytic lymphoma, prolymphocytic lymphoma, splenic marginal zone lymphoma, and HCLv [3, 8]. The typical phenotype of HCLc is co-expression of CD20, CD22, and CD11c; HCLc also shows expression of CD103 and CD25. CD5 expression is almost always negative in HCLc [3, 9]. Until recently, the genetic alterations underlying HCLc remained obscure. The scarcity of leukemic cells available for analysis because of pancytopenia and the very low proliferative index increased the difficulty level of molecular characterization of HCLc [4].
BRAF mutations activate the MEK.ERK pathway, leading to enhanced cell proliferation, cell survival, and neoplastic transformation [10, 11], and have emerged as an important biological marker for various human cancers, including papillary thyroid carcinoma and cutaneous malignant melanoma [5]. While over 70 BRAF mutations have been identified, V600E is predominant in many types of cancers. Very recently, the BRAF V600E mutation was reported in all cases of HCLc, but not in other B-cell neoplasms [4]. Subsequent studies confirmed this finding, reporting that all cases of HCLc examined carried BRAF V600E mutations and that mutations other than V600E were not obser-ved [12-14]. However, Xi et al. [15] reported that 21% of HCLc lack this mutation.
For detection of BRAF mutation, the use of a highly sensitive and specific method is essential, and the results can differ depending on the detection method used [5]. In the study by Tiacci et al. [4], the BRAF V600E mutation was detected by direct sequencing after Ficoll density gradient-enriched magnetic-activated cell sorting, and subsequent studies used more sensitive assays such as high-resolution melting [13], allele-specific real-time PCR [7], and pyrosequencing [6]. In the present study, we used a BRAF V600E-specific real-time PCR method that has been widely used for patients with papillary thyroid carcinoma and has shown higher sensitivity than dual priming oligonucleotide-based multiplex PCR [16, 17]. In addition to assay sensitivity, the type of specimen used should be considered in the detection of the BRAF V600E mutation in HCLc. Previous studies used various specimen types, including peripheral bloods, bone marrow biopsy specimens, and bone marrow aspirates [4, 6, 7, 12, 15, 18].
Bone marrow fibrosis is one of the typical findings in HCLc and often generates suboptimal aspirate specimens containing few malignant cells despite the extensive involvement observed during the biopsy [13]. In the present case, extensive reticulin fibrosis made bone marrow aspiration very difficult. The aspirate specimens for flow cytometry and molecular studies contained few hairy cells. The BRAF V600E mutation was first analyzed in bone marrow aspirates along with the other diagnostic tests, but it was not detected in these specimens. Because the diagnosis of HCLc was straightforward in this case, except for the negative finding for BRAF V600E mutation, we searched for BRAF V600E mutation again by using both right and left biopsy specimens; this time, we were able to detect it. The present case underscores that severe reticulin fibrosis of HCLc may generate discrepant results of BRAF V600E mutation in different types of specimens and may mislead diagnosis. Thus, the diagnostic interpretation of this new molecular marker in HCLc cases with extensive reticulin fibrosis warrants caution.
References
1. Sherman MJ, Hanson CA, Hoyer JD. An assessment of the usefulness of immunohistochemical stains in the diagnosis of hairy cell leukemia. Am J Clin Pathol. 2011; 136:390–399. PMID: 21846914.

2. Stetler-Stevenson M, Tembhare PR. Diagnosis of hairy cell leukemia by flow cytometry. Leuk Lymphoma. 2011; 52(Suppl 2):11–13. PMID: 21504292.

3. Summers TA, Jaffe ES. Hairy cell leukemia diagnostic criteria and differential diagnosis. Leuk Lymphoma. 2011; 52(Suppl 2):6–10. PMID: 21417827.

4. Tiacci E, Trifonov V, Schiavoni G, Holmes A, Kern W, Martelli MP, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011; 364:2305–2315. PMID: 21663470.
5. Ziai J, Hui P. BRAF mutation testing in clinical practice. Expert Rev Mol Diagn. 2012; 12:127–138. PMID: 22369373.
6. Verma S, Greaves WO, Ravandi F, Reddy N, Bueso-Ramos CE, O'Brien S, et al. Rapid detection and quantitation of BRAF mutations in hairy cell leukemia using a sensitive pyrosequencing assay. Am J Clin Pathol. 2012; 138:153–156. PMID: 22706871.
7. Schnittger S, Bacher U, Haferlach T, Wendland N, Ulke M, Dicker F, et al. Development and validation of a real-time quantification assay to detect and monitor BRAFV600E mutations in hairy cell leukemia. Blood. 2012; 119:3151–3154. PMID: 22331186.
8. Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Thiele J, et al. WHO classification of tumours of hematopoietic and lymphoid tissues. Lyon: IARC Press;2008.
9. Tiacci E, Liso A, Piris M, Falini B. Evolving concepts in the pathogenesis of hairy-cell leukaemia. Nat Rev Cancer. 2006; 6:437–448. PMID: 16723990.

10. Niault TS, Baccarini M. Targets of Raf in tumorigenesis. Carcinogenesis. 2010; 31:1165–1174. PMID: 20047953.

11. Li Y, Nakamura M, Kakudo K. Targeting of the BRAF gene in papillary thyroid carcinoma (review). Oncol Rep. 2009; 22:671–681. PMID: 19724843.
12. Arcaini L, Zibellini S, Boveri E, Riboni R, Rattotti S, Varettoni M, et al. The BRAF V600E mutation in hairy cell leukemia and other mature B-cell neoplasms. Blood. 2012; 119:188–191. PMID: 22072557.
13. Blombery PA, Wong SQ, Hewitt CA, Dobrovic A, Maxwell EL, Juneja S, et al. Detection of BRAF mutations in patients with hairy cell leukemia and related lymphoproliferative disorders. Haematologica. 2012; 97:780–783. PMID: 22133769.
14. Ping N, Wang Q, Wang Q, Dong S, Wu L, Xue Y, et al. Absence of BRAF V600E mutation in hematologic malignancies excluding hairy-cell leukemia. Leuk Lymphoma. 2012; 53:2498–2499. PMID: 22639828.
15. Xi L, Arons E, Navarro W, Calvo KR, Stetler-Stevenson M, Raffeld M, et al. Both variant and IGHV4-34-expressing hairy cell leukemia lack the BRAF V600E mutation. Blood. 2012; 119:3330–3332. PMID: 22210875.
16. Kwak JY, Jeong JJ, Kang SW, Park S, Choi JR, Park SJ, et al. Study of peripheral BRAF(V600E) mutation as a possible novel marker for papillary thyroid carcinomas. Head Neck. 2012; doi: 10.1002/hed.23195.
17. Kwak JY, Han KH, Yoon JH, Kim EK, Moon HJ, Kim YL, et al. BRAF V600E mutation testing in fine needle aspirates of thyroid nodules: potential value of real-time PCR. Ann Clin Lab Sci. 2012; 42:258–265. PMID: 22964613.
18. Tiacci E, Schiavoni G, Forconi F, Santi A, Trentin L, Ambrosetti A, et al. Simple genetic diagnosis of hairy cell leukemia by sensitive detection of the BRAF-V600E mutation. Blood. 2012; 119:192–195. PMID: 22028477.
Fig. 1
(A) Bone marrow aspirate smear showing characteristic hairy cells with cytoplasmic projections and frayed cytoplasmic border (Wright-Giemsa stain, ×1,000). (B) Bone marrow biopsy section showing diffuse solid infiltration of medium-sized lymphoid cells with abundant cytoplasm (fried-egg appearance) (H&E stain, ×400). (C) Extensive reticulin fibrosis (Reticulin stain, ×400). (D) Strong positivity for CD20 (CD 20 immunostain, ×200).
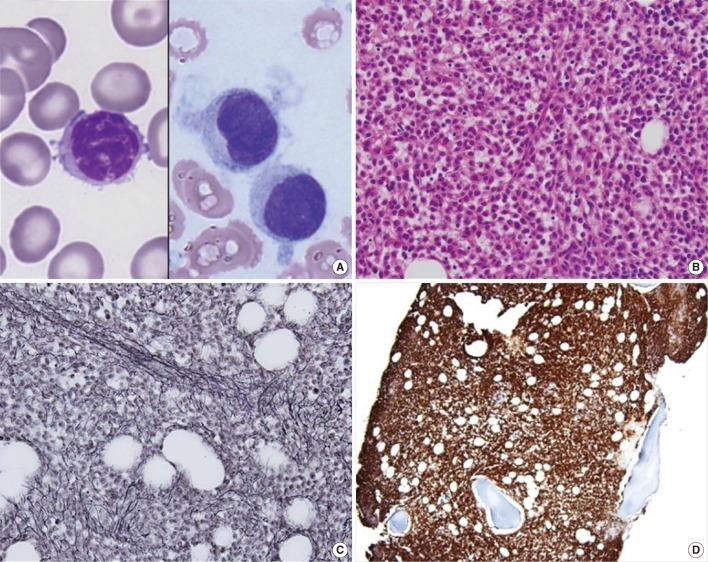
Fig. 2
Immunophenotyping of bone marrow aspirate shows abnormal lymphoid cells positive for CD19, CD20, and CD11c and negative for CD5 and CD10. HLA-DR, cytoplasmic CD79a, and CD2 were also positive, and other T-lymphoid (CD7, CD3, and cCD3) and myeloid markers (CD13, CD33, myeloperoxidase and CD14) were negative (not shown).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download