Klinefelter syndrome (KS), with an incidence of 1 in 600 male newborns, is the most common type of X chromosome aneuploidy. Individuals with KS are characterized by tall stature, decreased secondary sexual characteristics, small testicles, gynecomastia, and infertility. About 80% of patients have the karyotype 47,XXY [1]. An extra X chromosome as a sole acquired abnormality has also been reported in patients with several hematologic malignancies such as acute lymphoblastic leukemia [2], AML [3, 4], and chronic neutrophilic leukemia [5]. It has been suggested that an extra X chromosome has high oncogenic potential, predisposing the carriers to leukemia. In practice, it is sometimes difficult to distinguish whether the extra X chromosome is constitutional or acquired in a patient with hematologic malignancy. Here, we describe a mosaic KS patient who was diagnosed with primary myelofibrosis, which progressed to AML. Because of the obscure clinical phenotype, we originally reported his sex chromosome aneuploidy as an acquired anomaly secondary to his hematologic malignancy.
A 62-yr-old man presented with pancytopenia with sustained fatigue, poor general condition, and excessive weight loss (10 kg in 2 months). His complete blood count revealed the followings: hemoglobin, 7.4 g/dL; leukocyte count, 3.45×109/L (absolute neutrophil count, 0.93×109/L); and platelets, 42×109/L. In addition, analysis of the peripheral blood revealed 3% myeloblasts and leukoerythroblastic features. The patient had mild splenomegaly on the abdominal computed tomography scan. For further evaluation, the patient underwent bone marrow aspiration and biopsy, and the results suggested primary myelofibrosis (PMF), fibrotic stage, with diffuse bone marrow fibrosis that was evident on Masson-trichrome and reticulin staining. The estimated cellularity of the bone marrow section was 100%. JAK2 V617F mutation analysis was negative. Conventional cytogenetic evaluation of the peripheral blood lymphocyte culture using conventional G-banding revealed 2 cell lines, 47,XXY and 46,XY, with the dominant karyotype being 47,XXY (Fig. 1). Clinical investigation revealed normal-sized testes, masculine pubic and axillary hair, no gynecomastia, average height (174 cm) and weight (75 kg), and a normal serum testosterone level (0.75 µIU/mL; cut-off level, 0.35-5.5 µIU/mL). The patient was married and fathered a child, inconsistent with KS. Therefore, we reported the karyotype of 47,XY,+X[14]/46,XY[2] as an acquired anomaly rather than a constitutional abnormality. Two months later, the blasts in the patient's blood increased up to 47%, and marrow examination revealed a packed marrow with myeloperoxidase (MPO)-positive blasts and diffuse fibrosis, as observed before. Flow cytometric immunophenotyping revealed blasts positive for CD33, CD117, CD11c, CD64, CD56, and MPO. These findings were consistent with a diagnosis of AML with monocytic differentiation. At the time of AML transformation, the chromosome analysis still showed the 47,XXY karyotype in all 23 metaphase cells analyzed. After induction chemotherapy, the patient achieved complete remission; however, the karyotype abnormality did not disappear.
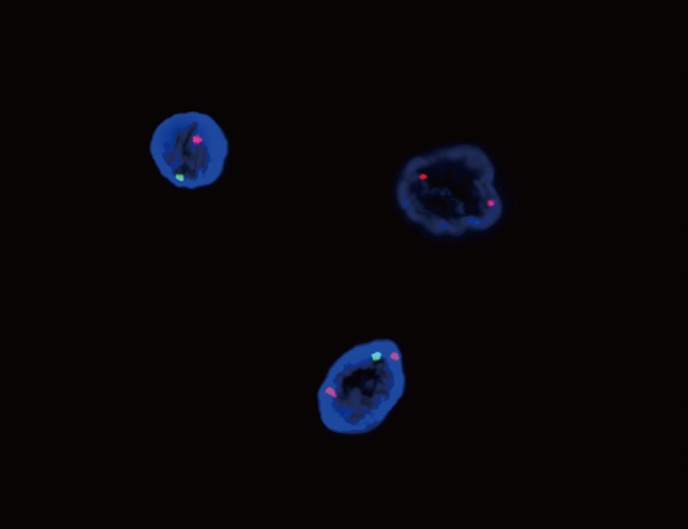
To evaluate the persistent cytogenetic abnormalities, we further investigated lymphocytes and buccal mucosa cells using sex chromosome-specific probes that targeted the α-satellite of the X centromere region and satellite III of Yq12 (CEP X, Spectrum Orange; CEP Y, Spectrum Green) as described by the manufacturer (Abbott Molecular, Abbot Park, IL, USA). These probes were hybridized to interphase cells and visualized by fluorescence microscopy using FISH. The number of individual cells was expressed as a percentage of the total number of interphase cells analyzed. A specimen that contains >2.30% of cells with a signal pattern other than XY in male patients was considered to have an abnormal complement of sex chromosomes. The FISH results for 200 interphase nuclei from buccal smear cells were as follows: 86% XXY, 11.5% XY, and 2.5% XX (Fig. 2). With the exception of the XX signals, the proportions of XXY and XY signals in lymphocytes were similar to those observed in buccal cells (91.2% and 8.8%, respectively). The XX signal pattern observed in the buccal smear cells was probably due to the artificial loss of 1 Y chromosome during the FISH procedure or true mosaicism with 3 cell lines [6]. Consequently, the patient was diagnosed with mosaic KS.
The classical phenotype of KS is widely recognized, but some affected individuals have no discrete clinical features, especially in mosaic KS. They may have normal-sized testes and less severe endocrine abnormalities; further, they may be fertile because of the presence of some normal clones of cells within the testes, as observed in our case [7]. The variation in phenotype most likely depends on the number of abnormal cells and their location in the body [8]. Consequently, the disorder might be underdiagnosed; only approximately 25% of adult men with KS are diagnosed [9], and the referring primary or secondary centers do not suspect 60% of KS patients to have the disorder, despite previous clinical investigations [10].
There are several reports of hematologic malignancies with unusual, sole X chromosome aberrations [2-5, 11-13]. These observations suggest that we should be careful when concluding whether an abnormality is constitutional or acquired, especially when the patient has a hematologic malignancy. To prevent misinterpretation, it can be helpful to perform serial cytogenetic evaluation. If an initial abnormal karyotype returns to a normal female or male karyotype after chemotherapy, then the initial cytogenetic abnormality represents an acquired aberration rather than a constitutional one. Careful clinical investigation should be performed in every case, although general features should be regarded with caution because patients with chromosomal mosaicism commonly show very few clinical symptoms, as described in this case. Another approach can be the use of complementary diagnostic methods, such as interphase FISH. Although cytogenetic analysis of peripheral blood lymphocytes is the gold standard to confirm KS, interphase FISH for different somatic cell lines (for example, buccal cells, skin fibroblasts, or testicular biopsy samples) can be used to confirm chromosomal mosaicism when cytogenetic analysis of peripheral blood reveals a normal male karyotype in a patient with suspected KS [7]. FISH is more accurate in determining the exact number of sex chromosomes, defining the cytogenetic status as mosaic or nonmosaic, and assessing the ratios of cell populations in mosaicism [14]. In conclusion, we described a mosaic KS patient who had PMF with AML transformation. Chromosomal mosaicism was confirmed by additional FISH analysis of the buccal smear cells. We suggest that interphase FISH should be performed in different somatic cells in order to determine the cytogenetic status of a patient with suspected KS, especially when an extra X chromosome is the only abnormality.
References
1. Wikström AM, Dunkel L. Klinefelter syndrome. Best Pract Res Clin Endocrinol Metab. 2011; 25:239–250. PMID: 21397196.

2. Yamamoto K, Hato A, Minagawa K, Yakushijin K, Urahama N, Sada A, et al. An extra X chromosome as a sole abnormality in relapse of an adult acute lymphoblastic leukemia. Cancer Genet Cytogenet. 2004; 155:154–155. PMID: 15571803.

3. Tsutsumi Y, Tanaka J, Minami H, Musashi M, Fukushima A, Ehira N, et al. Acute biphenotypic leukemia and an acquired X chromosome. Cancer Genet Cytogenet. 2005; 157:94–95. PMID: 15676158.

4. Wan TS, Yip SF, Yeung YM, Chan LC, Ma SK. Fatal diffuse alveolar damage complicating acute myeloid leukemia with abnormal eosinophils and trisomy X. Ann Hematol. 2002; 81:167–169. PMID: 11904745.

5. Yamamoto K, Nagata K, Kida A, Hamaguchi H. Acquired gain of an X chromosome as the sole abnormality in the blast crisis of chronic neutrophilic leukemia. Cancer Genet Cytogenet. 2002; 134:84–87. PMID: 11996803.

6. Hur M, Cho HC, Lee KM, Park H, Lee SY, Kim KN, et al. Cleft palate in a rare case of Variant Klinefelter syndrome with 48,XXXY/46,XY mosaicism. Cleft Palate Craniofac J. 2009; 46:555–557. PMID: 19929089.

7. Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter's syndrome. Lancet. 2004; 364:273–283. PMID: 15262106.

8. Kruse R, Guttenbach M, Schartmann B, Schubert R, van der Ven H, Schmid M, et al. Genetic counseling in a patient with XXY/XXXY/XY mosaic Klinefelter's syndrome: estimate of sex chromosome aberrations in sperm before intracytoplasmic sperm injection. Fertil Steril. 1998; 69:482–485. PMID: 9531882.

9. Abramsky L, Chapple J. 47,XXY (Klinefelter syndrome) and 47,XYY: estimated rates of and indication for postnatal diagnosis with implications for prenatal counselling. Prenat Diagn. 1997; 17:363–368. PMID: 9160389.

10. Kamischke A, Baumgardt A, Horst J, Nieschlag E. Clinical and diagnostic features of patients with suspected Klinefelter syndrome. J Androl. 2003; 24:41–48. PMID: 12514081.
11. Zeidan A, Phatak P. Acquired biclonal chromosome X aberrations without autosomal chromosomal anomalies in acute myeloid leukemia. Cancer Genet Cytogenet. 2008; 181:125–130. PMID: 18295665.

12. Yhim HY, Kim HS, Sohn JY, Song MJ, Lee NR, Song EK, et al. JAK2 V617F-positive essential thrombocythemia in a patient with Klinefelter syndrome: a case report. Cancer Genet Cytogenet. 2010; 198:162–165. PMID: 20362232.

13. Kumar S, Menke DM, Dewald GW, Colon-Otero G. Agnogenic myeloid metaplasia associated with Klinefelter syndrome: a case report. Ann Hematol. 2002; 81:215–218. PMID: 11976824.
14. Okada H, Dobashi M, Yamazaki T, Fujisawa M, Arakawa S, Kamidono S. Fluorescence in situ hybridization analysis of sex-chromosome mosaicism in azoospermic men. J Androl. 2001; 22:970–972. PMID: 11700861.

Fig. 1
Karyotyping of peripheral blood cells using conventional G-banding revealed an extra X chromosome.
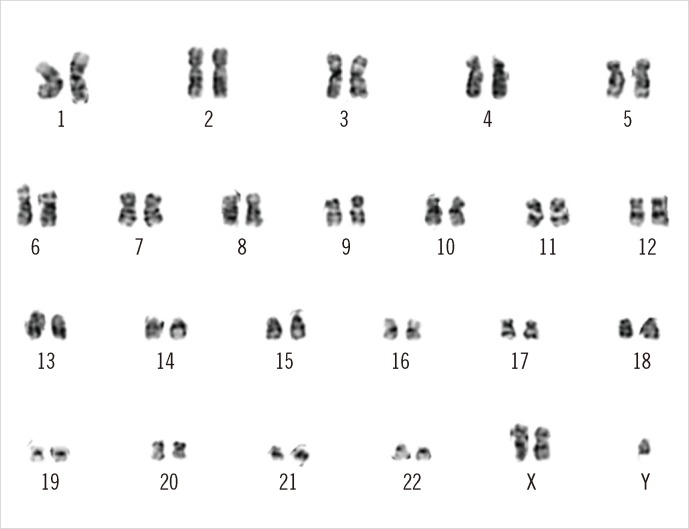
Fig. 2
FISH analyses using sex chromosome-specific probes to the α-satellite of the X centromere region (red signal) and satellite III of Yq12 (green signal) on a buccal smear specimen revealed 86% XXY and 11.5% XY. The remaining 2.5% of cells had an XX signal pattern, probably due to the artificial loss of 1 Y chromosome in the FISH procedure or true mosaicism with 3 cell lines.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download