Abstract
Recently, lyso-globotriaosylsphingosine (lyso-Gb3) was found to be elevated in plasma of treatment naive male patients and some female patients with Fabry Disease (FD). This study tested whether lyso-Gb3 could be analyzed in dried blood spots (DBS) from filter cards and whether concentrations are elevated in newborn infants with FD. Lyso-Gb3 concentrations were analyzed in DBS following extraction using a novel HPLC-mass spectrometry (MS)/MS method. Lyso-Gb3 levels in DBS were above the lower limit of quantitation (0.28 ng/mL) in 5/17 newborn FD infants (16 males; range: 1.02-8.81 ng/mL), but in none of the newborn controls, in all 13 patients (4 males) with classic FD (range: 2.06-54.1 ng/mL), in 125/159 Taiwanese individuals with symptomatic or asymptomatic FD who carry the late onset α-galactosidase A (GLA) mutation c.936+919G>A (IVS4+919G>A) (3.75±0.69 ng/mL; range: 0.418-3.97 ng/mL) and in 20/29 healthy controls (0.77±0.24 ng/mL; range: 0.507-1.4 ng/mL). The HPLC-MS/MS method for analysis of lyso-Gb3 is robust and yields reproducible results in DBS in patients with FD. However, concentrations of lyso-Gb3 were below the limit of quantitation in most newborn infants with FD rendering this approach not suitable for newborn screening. In addition, most females with the late onset mutation have undetectable lyso-Gb3 concentrations.
Fabry disease (FD, OMIM #301500) is a X-linked inherited lysosomal storage disorder caused by the deficiency of α-galactosidase A (GLA, E.C.3.2.1.22) that leads to progressive storage of neutral glycosphingolipids in vascular endothelium, smooth muscle cells, podocytes and other tissues [1]. The diagnosis in affected hemizygotes can be readily achieved by GLA analysis and/or molecular analysis [1, 2]. However, it is well established that a significant number of affected FD heterozygotes may have GLA activities that fall within the normal range rendering enzyme diagnosis unreliable [2-4].
Analysis of globotriaosylceramide and its isoforms in urine or in plasma may aid in the diagnosis of FD [5-7]. Recently, the deacylated form of globotriaosylceramide, lyso-globotriaosylsphingosine (lyso-Gb3) was shown to be significantly elevated in plasma of males and to a lesser extent in plasma of symptomatic females with classic FD [7, 8]. Despite limited data, lyso-Gb3 may be useful as a biomarker which appears to inhibit GLA activity and to promote smooth muscle cell proliferation explaining some but not all of the pathological features of FD [7-9].
We have developed a highly sensitive HPLC tandem mass spectrometry (MS/MS) method for the analysis of lyso-Gb3 in dried blood spots (DBS). Using this method we investigated whether lyso-Gb3 is elevated in DBS of newborn infants with FD from the Taiwanese Fabry Newborn Screening Program [10]. In addition, we tested whether lyso-Gb3 may serve as a disease marker in females with symptomatic or asymptomatic FD, particularly in those with the late onset GLA mutation c.936+919G>A (IVS4+919G>A) [10].
Seventeen Taiwanese newborn infants with FD (16 males), who were identified through the Taiwanese Fabry Newborn Screening Program, were included in the study. Gender, age of diagnosis, GLA activities in DBS and leukocytes, as well as the GLA mutations are listed in Table 1A. An additional 172 individuals with FD including 159 Taiwanese subjects (mean age: 21.19 yr; range: 0.11-87.73 yr; 79 males), who carry the late-onset mutation c.936+919G>A (IVS4+919G>A) were studied [10]. The remaining 13 individuals with FD were of Austrian or Hungarian origin (Table 1B). Seventeen Taiwanese newborn infants with different inborn errors of metabolism (Phenylketonuria N=16; 3-Methylcrotonyl Carboxylase deficiency N=1; 16 males; age: 1.03 months; range: 0.47-3.30 months) and 29 non-affected family members of the Taiwanese FD patients were studied as controls. The study was approved by the research Ethics Committee of the National Taiwan University Hospital (NTUH-Rec 200711033R).
Lyso-ceramide trihexoside (lyso-globotriaosylsphingosine, lyso-Gb3) and lyso-lactosylceramide (lyso-Gb2, used as internal standard) (Matreya LLC; Pleasant Gap, PA, USA) were of >98% purity. 1 mg of each reference standard were dissolved in 2 mL of dimethyl sulfoxide (DMSO)/methanol (1/1=v/v), separately to make stock solutions. Internal standard working solution was prepared from 410 µL of its stock solution and addition of 500 mL of ethanol (400 ng/mL lyso-Gb2). For lyso-Gb3, 8.16 µL of the stock solution was diluted up to 5 mL (0.800 µg/mL) with DMSO/methanol (1/1=v/v). All calibration standards were spiked in 50% methanol at concentrations of 0.410/1.06/2.59/6.15/15.3/41.0/102 ng/mL lyso-Gb3.
A gradient HPLC method on a reversed phase column (ACE 3 C8, 50×2.1 mm, 3 µm, Advanced Chromatography Technology; Aberdeen, Scotland) was used on an API 4000 Q-Trap mass spectrometer (Applied Biosystems; Carlsbad, CA, USA) equipped with 2 HPLC pumps and a column oven (Perkin Elmer; Waltham, MA, USA) set at 60℃. Flow rate was set at 0.9 mL/min. The mobile phases were 50 mM formic acid in water (A) and 50 mM formic acid in acetonitrile/acetone (1/1=v/v; B). A linear gradient starting at 5% B up to 66% B from 0 to 4.0 min and isocratic conditions from 4.1 to 5.1 min at 100% B were used. Re-equilibration was done from 5.1 to 5.9 min at 5% B. The injection volume was 5 µL.
Electrospray ionization in positive mode was used for peak detection. Multiple Reaction Monitoring (MRM) with a vaporizer temperature of 500℃, ionization voltage of 5.5 kV, curtain gas pressure of 40 psi, gas 1 pressure of 45 psi, and gas 2 pressure of 60 psi, declustering potential of 40 V, and "low" collisionally activated dissociation gas was used. Lyso-Gb3 MRM transition was 786.6>282.2 m/z and lyso-Gb2 MRM transition was 624.5>282.2 m/z. Fig. 1 shows an example of a chromatogram in a healthy control subject and an individual with FD.
3 mm DBS (~3 µL of whole blood; [11, 12] were punched from a filter card, mixed with 75 µL internal standard working solution, and put into an ultrasonic bath for 5 min before centrifugation. The clear supernatant was transferred into auto sampler vials.
Statistical analyses were carried out using R statistical software v2.10.1 (Revolution analytics, Palo Alto, CA, USA). A Wilcoxon rank sum test was used for all two sample comparisons, while the Kruskal-Wallis test was used for three or more sample comparisons. Post hoc testing was performed using the Nemenyi-Damico-Wolfe-Dunn test. Significance was assumed when P<0.05.
Lyso-Gb3 in DBS was below the LLOQ (0.28 ng/mL) in all of the newborn controls, but was elevated in 5/17 newborn infants with FD (range: 1.02-8.81 ng/mL). However, lyso-Gb3 levels in newborn infants with FD were not statistically different from lyso-Gb3 levels in newborn controls (P=0.189). In contrast, lyso-Gb3 was detectable in DBS in all 13 older patients (4 males) with classic FD (range: 2.06-54.1 ng/mL) and these levels in both older males (mean 41.5±13.36 ng/mL) and females (mean 3.5±1.86 ng/mL) with classic FD were statistically higher than in newborns with FD (mean 1.12±2.10 ng/mL; Kruskal-Wallis, P=1.154×10-4; Post hoc testing: classic males vs. classic females P=0.871, classic males vs. newborns P=1.11×10-5, classic females vs. newborns P=0.002; Fig. 2A). These results suggest that lyso-Gb3 may not be a good marker for FD in Taiwanese newborns.
Lyso-Gb3 in DBS was detected in 125/159 untreated Taiwanese patients with symptomatic or asymptomatic FD who carry the late onset GLA mutation c.936+919G>A (IVS4+919G>A) (3.75±0.69 ng/mL, range: 0.418-3.97 ng/mL for the 125 patients with detectable lyso-Gb3). Lyso-Gb3 was above the lower limit of quantitation in 20/29 healthy Taiwanese control subjects (0.77±0.24 ng/mL, range: 0.507-1.4 ng/mL). Lyso-Gb3 levels were not statistically different between the Taiwanese healthy controls (mean 0.57±0.31 ng/mL) and both males (mean 0.95±0.88 ng/mL) and females (mean 0.67±0.31 ng/mL) who carry the late onset GLA mutation (P=0.159, Fig. 2B). However, FD individuals that have the late onset mutation have reduced levels of lyso-Gb3 compared to classic FD patients (P=7.756×10-8; Fig. 2C).
Plasma lyso-Gb3 levels are markedly elevated in both symptomatic heterozygous and hemizygous patients with classic FD and may be consequently used as a diagnostic marker for classic FD [7, 8]. In addition, lyso-Gb3 levels were within normal limits in patients with classic FD following 3 months of enzyme replacement therapy using either agalsidase alpha or beta respectively [13].
Lyso-Gb3 in DBS was markedly elevated in the limited number of both male and female patients with classical FD studied, comparable to the findings reported previously for plasma [7, 14]. Lyso-Gb3 levels may be normal in younger females with pre-symptomatic FD [7]. Due to the lack of information on the clinical phenotype we were not able to correlate clinical findings with lyso-Gb 3 levels.
In conclusion, lyso-Gb3 can be readily measured in DBS using this robust and sensitive analytical technique. Analysis of lyso-Gb3 in DBS may be an important asset for high-throughput screening for classical FD in at-risk populations. However, its utility for newborn screening is very limited. Additional population-based studies are needed to further validate this method.
References
2. Gal A, Hughes DA, Winchester B. Toward a consensus in the laboratory diagnostics of Fabry disease - recommendations of a European expert group. J Inherit Metab Dis. 2011; 34:509–514. PMID: 21229318.

3. Dajnoki A, Fekete G, Keutzer J, Orsini JJ, De Jesus VR, Chien YH, et al. Newborn screening for Fabry disease by measuring GLA activity using tandem mass spectrometry. Clin Chim Acta. 2010; 411:1428–1431. PMID: 20338160.

4. MacDermot KD, Holmes A, Miners AH. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet. 2001; 38:769–775. PMID: 11732485.

5. Fauler G, Rechberger GN, Devrnja D, Erwa W, Plecko B, Kotanko P, et al. Rapid determination of urinary globotriaosylceramide isoform profiles by electrospray ionization mass spectrometry using stearoyl-d35-globotriaosylceramide as internal standard. Rapid Commun Mass Spectrom. 2005; 19:1499–1506. PMID: 15880667.

6. Paschke E, Fauler G, Winkler H, Schlagenhauf A, Plecko B, Erwa W, et al. Urinary total globotriaosylceramide and isoforms to identify women with Fabry disease: a diagnostic test study. Am J Kidney Dis. 2011; 57:673–681. PMID: 21186071.

7. Rombach SM, Dekker N, Bouwman MG, Linthorst GE, Zwinderman AH, Wijburg FA, et al. Plasma globotriaosylsphingosine: diagnostic value and relation to clinical manifestations of Fabry disease. Biochim Biophys Acta. 2010; 1802:741–748. PMID: 20471476.

8. Aerts JM, Groener JE, Kuiper S, Donker-Koopman WE, Strijland A, Ottenhoff R, et al. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A. 2008; 105:2812–2817. PMID: 18287059.

9. Kalliokoski RJ, Kalliokoski KK, Penttinen M, Kantola I, Leino A, Viikari JS, et al. Structural and functional changes in peripheral vasculature of Fabry patients. J Inherit Metab Dis. 2006; 29:660–666. PMID: 16906474.

10. Hwu WL, Chien YH, Lee NC, Chiang SC, Dobrovolny R, Huang AC, et al. Newborn screening for Fabry disease in Taiwan reveals a high incidence of the later-onset GLA mutation c.936+919G>A (IVS4+919G>A). Hum Mutat. 2009; 30:1397–1405. PMID: 19621417.
11. Adam BW, Alexander JR, Smith SJ, Chace DH, Loeber JG, Elvers LH, et al. Recoveries of phenylalanine from two sets of dried-blood-spot reference materials: prediction from hematocrit, spot volume, and paper matrix. Clin Chem. 2000; 46:126–128. PMID: 10620584.

12. Chace DH, Adam BW, Smith SJ, Alexander JR, Hillman SL, Hannon WH. Validation of accuracy-based amino acid reference materials in dried-blood spots by tandem mass spectrometry for newborn screening assays. Clin Chem. 1999; 45:1269–1277. PMID: 10430794.

13. van Breemen MJ, Rombach SM, Dekker N, Poorthuis BJ, Linthorst GE, Zwinderman AH, et al. Reduction of elevated plasma globotriaosylsphingosine in patients with classic Fabry disease following enzyme replacement therapy. Biochim Biophys Acta. 2011; 1812:70–76. PMID: 20851180.

14. Togawa T, Kodama T, Suzuki T, Sugawara K, Tsukimura T, Ohashi T, et al. Plasma globotriaosylsphingosine as a biomarker of Fabry disease. Mol Genet Metab. 2010; 100:257–261. PMID: 20409739.

Fig. 1
Representative chromatograms from a normal control individual (A) and a male with Fabry Disease (FD) (B). Increased levels of lyso-globotriaosylsphingosine (lyso-Gb3) are detected in males with FD.
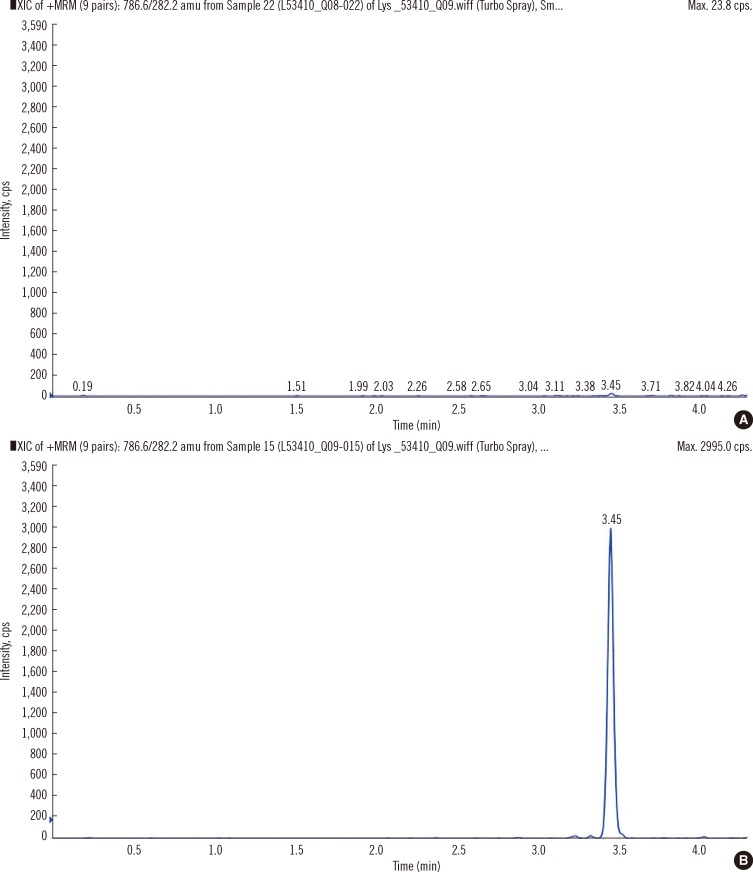
Fig. 2
(A) Lyso-Gb3 levels in classical FD patients and newborns with FD. Classic FD patients have higher levels of lyso-Gb3 than newborns with FD (Kruskal-Wallis, P=1.154×10-4). (B) Lyso-Gb3 levels in healthy controls and late onset FD patients. Lyso-Gb3 levels are not statistically different between healthy controls and the Taiwanese patients with symptomatic or asymptomatic FD, who carry the late onset GLA mutation c.936+919G>A (IVS4+919G>A; Kruskal-Wallis, P=0.159). (C) Lyso-Gb3 levels in classic FD patients and late onset FD patients. Patients with classic FD have higher lyso-Gb3 levels than the Taiwanese patients with symptomatic or asymptomatic FD, who carry the late onset GLA mutation c.936+919G>A (IVS4+919G>A; Wilcoxon, P=7.756×10-8).
Abbreviations: Lyso-Gb3, lyso-globotriaosylsphingosine; FD, Fabry Disease; LLOQ, Lower limit of quantitation; GLA, α-galactosidase A.
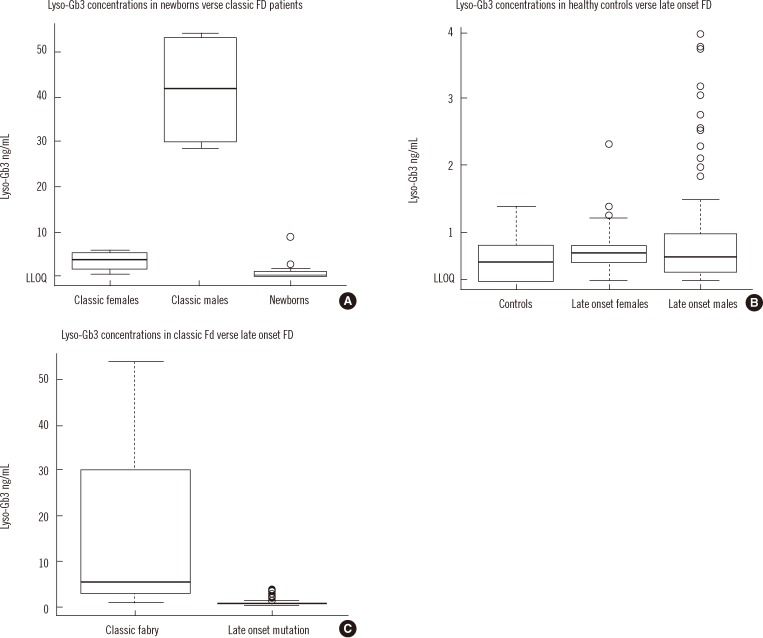
Table 1
Characteristics of the Taiwanese newborn infants (A) and the Hungarian and Austrian adults with FD (B)
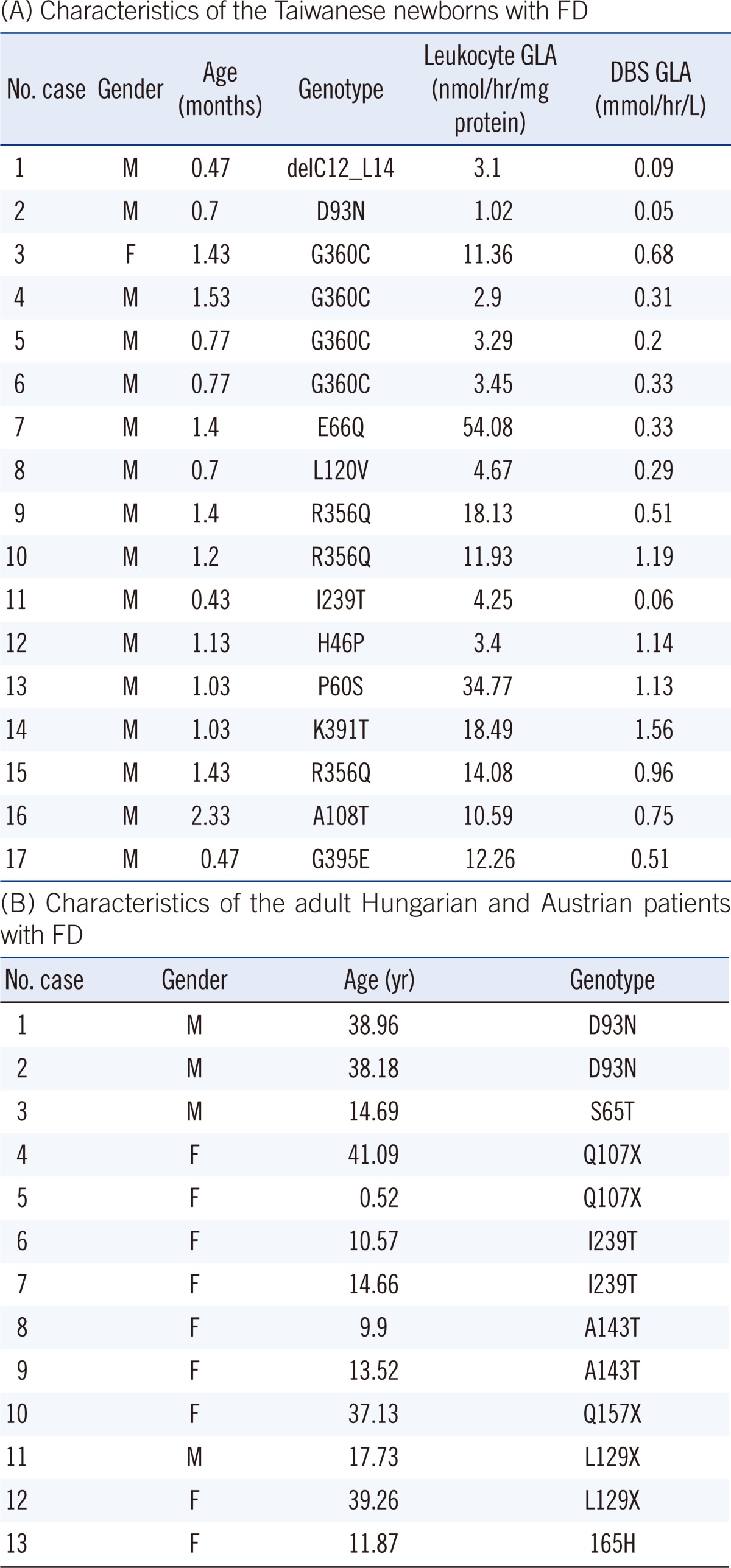
(A) Age, gender, GLA activities in dried blood spots (reference range: 5.87±3.04 µmol/hr/L) and leukocytes (reference range: 95.13±30.3 nmol/hr/mg protein), and genotypes were included.
(B) Patient 4 (mother) and 5 (daughter) are related; patients 6 and 7 are sisters as are patients 8 and 9. Patient 12 is the mother of patient 11. Patients 4, 11, and 12 are on enzyme replacement therapy.
Abbreviations: M, male; F, female; FD, Fabry Disease; DBS, Dried Blood Spot; GLA, α-galactosidase A.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download