Abstract
Background
The Hb levels of prospective blood donors are usually determined using a finger prick test. A new noninvasive Hb device has the advantage of not causing any sampling pain. The purpose of this study was to evaluate the accuracy of the noninvasive Hb sensor and to compare its measurements with those of a currently used portable hemoglobinometer.
Methods
Hb was measured using a noninvasive Hb sensor (NBM-200; OrSense, Israel), a portable hemoglobinometer (HemoCue; HemoCue AB, Sweden), and an automated hematology analyzer (LH500; Beckman Coulter, USA). The correlations between Hb measurements taken by the NBM-200 and HemoCue with those by an automated hematology analyzer were assessed using intraclass correlation coefficients (ICCs). Hb measurements were compared among 3 different Hb level groups.
Results
The mean Hb values of 506 blood donors were 14.1 g/dL by the NBM-200, 14.0 g/dL by the LH500, and 14.3 g/dL by the HemoCue. The correlation between the LH500 and the NBM-200 was substantial (ICC=0.69), while that between the LH500 and the HemoCue agreed almost perfectly (ICC=0.86).
Conclusions
The possibility to judge to be eligible for donors who are ineligible to donate was substantial when using NBM-200. Even though the NBM-200 has the apparent advantage
of noninvasiveness, its use in pre-screening should be given meticulous attention. Since pre-donation testing is crucial to protecting donors' health, complete evaluation of the instrument should be performed prior to use.
Hb estimation is an integral part of donor screening to ensure donor safety and guarantee blood quality. The finger-stick CuSO4 method has been the pre-donation screening method of choice for several decades. This method is widely used because it is fast, cost-effective, and easy to perform. However, quality control of the copper sulfate solution is difficult, and it presents problems of biohazardous material disposal [1]. Even with strict quality control procedures in place, the CuSO4 method may fail to detect healthy donors or donors with abnormal protein levels and leukocytosis. The inaccuracy of the CuSO4 method has been continuously pointed out since it provides quantitative results and has a subjective endpoint [2-4]. Due to the unacceptable accuracy of the gravimetric CuSO4 method, it has increasingly been replaced with more accurate point-of-care testing (POCT) devices that measure either Hb or hematocrit, such as the HemoCue (HemoCue AB, Angelholm, Sweden), HemataSTAT II (Separation Technology Inc., Altamonte Springs, FL, USA), and BeneCheck (General Life Biotechnology Co., Ltd, Taipei, Taiwan).
The HemoCue, a portable hemoglobinometer that uses the dry chemistry principle, has been widely used to check Hb levels in pre-donation screenings. The HemoCue has been available for several years and gives precise and accurate results when used on venous blood under laboratory conditions [5, 6]. The cost of using the HemoCue is comparatively higher than that of the CuSO4 method. Although the popularity of the HemoCue is increasing, reports regarding its accuracy are conflicting.
The currently used methods that employ a copper sulfate solution and a portable hemoglobinometer require donors to prick their fingers, which can cause pain. The finger prick is reported by blood donors as one of the worst parts of the blood donation process. Eliminating a major source of discomfort for donors may improve donor satisfaction and increase their willingness to donate in the future.
A newly released noninvasive Hb measurement instrument, the NBM-200 (OrSense, Nes Ziona, Israel), takes optical measurements after temporary blood flow occlusion using a pneumatic finger cuff. Previous studies have validated the use of this noninvasive hemoglobinometer compared to multi-wavelength pulse CO-oximeters, but only in regards to patient care [5]. Lotfi et al. [7] reported that use of a noninvasive method of measuring post-donation Hb to determine donor eligibility saved time and expenditure without endangering blood donors. To date, no study that has assessed the accuracy of the noninvasive NBM-200 Hb sensor with regard to pre-donation screening, although its use has been described in other fields [8]. The objectives of the current study were to evaluate the accuracy of the NBM-200 and to compare it with that of the currently used HemoCue in the measurement of Hb as part of the blood donor screening process.
The study was conducted at 2 blood donation sites, both of which were affiliated to Hanmaum Blood Center (Gwacheon, Korea). Blood donation volunteers who agreed to participate in this study were enrolled between April and September 2011. Informed consent was obtained from all of the participants.
Five-milliliter venous samples were collected in EDTA vacutainers (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and the Hb was tested using an LH500 automated hematology analyzer (Beckman Coulter, Inc., Brea, CA, USA) to determine the reference values. Capillary blood for the Hb measurements taken using the HemoCue was obtained from the finger at the same time the blood samples were taken for laboratory analysis. The noninvasive NBM-200 Hb sensor was placed on the donor's thumb, and the Hb level was obtained within 1 min. The room temperature was kept at 18-26℃, and its humidity was kept at 30-70%.
The volunteers' systolic blood pressures were 90-180 mmHg, and their diastolic blood pressures were <100 mmHg. Three quality standard materials (low, normal, high) for the LH500 were measured every day, and tests were done only when the Hb values of the standard materials were within 3 SD. Control cuvette was used to monitor HemoCue accuracy. Hb level was assessed when Hb values of the control cuvette were within±0.3 g/dL of the assigned value.
Hb measurements obtained using the NBM-200 and HemoCue were compared with those obtained using the LH500. Using the LH500 values as a reference, the data were split into the following 3 groups since the eligibility criteria for an apheresis donation is ≥12.0 g/dL and that for a whole blood donation is ≥12.5 g/dL.
Group 1: Hb <12.0 g/dL
Group 2: Hb 12.0-12.4 g/dL
Group 3: Hb ≥12.5 g/dL
Hb measurements according to gender were compared among the 3 methods.
This study protocol was approved by the institutional review board of the Hanmaum Blood Center.
Sensitivity and specificity were calculated as the percentage of ineligible donors who were correctly identified as ineligible to donate blood and the percentage of eligible donors who were correctly identified as eligible to donate blood, respectively. The degree of homogeneity between the Hb measurements obtained using the 2 POCT devices (NBM-200, HemoCue) and the reference value obtained using the LH500 was evaluated by utilizing the intraclass correlation coefficient (ICC). Bland-Altman plots accommodating the double measurements within subjects were used to show the difference between the Hb measurements obtained using the 2 POCT devices (NBM-200, HemoCue) along the y-axis vs. the Hb measurements obtained using the LH500 along the x-axis with the 95% limits of agreement.
All statistical analyses were performed using SPSS Statistics 18.0 (SPSS Inc., Chicago, IL, USA). The ICCs were interpreted as follows: <0, poor; 0.01-0.20, slight agreement; 0.21-0.40, fair agreement; 0.41-0.60, moderate agreement; 0.61-0.80, substantial agreement; and 0.81-1.00, almost perfect agreement [9].
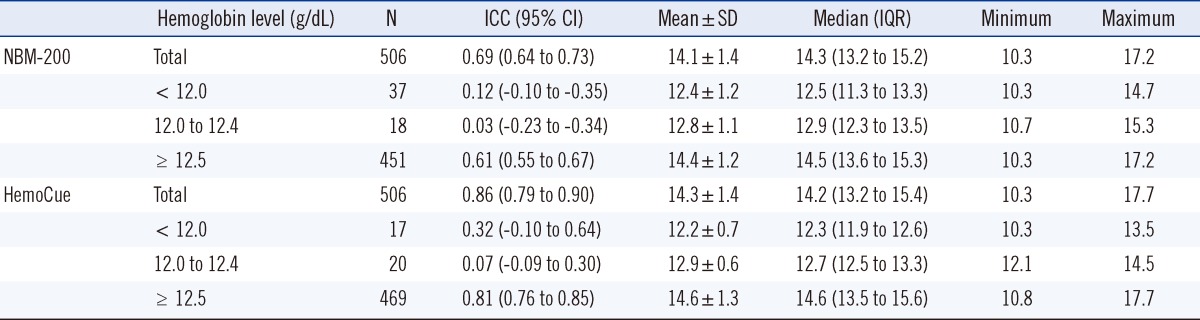
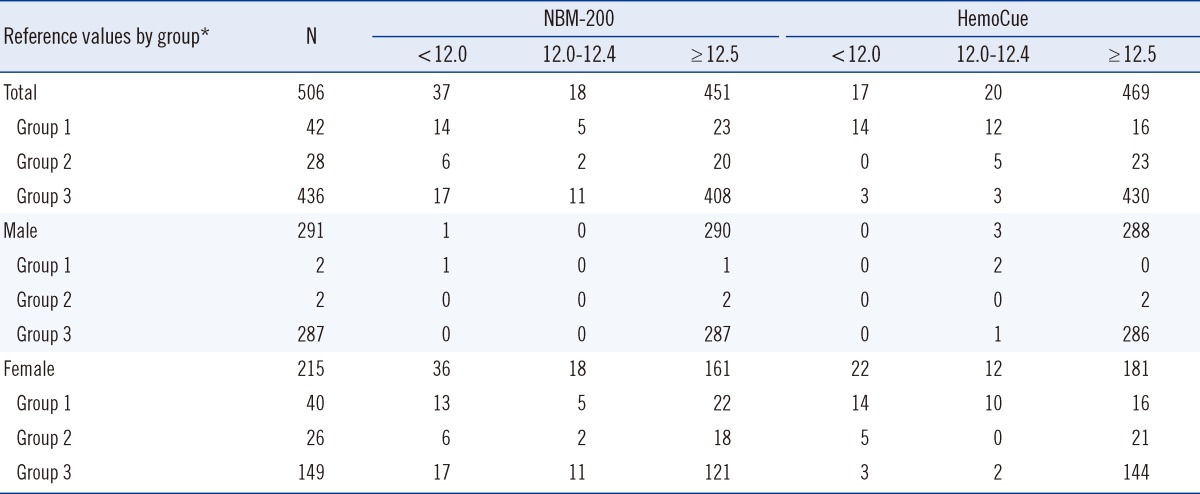
Hb was measured in 506 donors (291 men, 215 women). The average Hb measurements were 14.0, 14.1, and 14.3 g/dL using the LH500, NBM-200, and HemoCue, respectively (Table 1). The Hb measurements of the LH500 had a normal distribution, while those of the NBM-200 were left-skewed and those of HemoCue were slightly right-skewed (Fig. 1).
A scatter plot of the NBM-200 vs. the LH500 showed wider distribution than that of the HemoCue vs. the LH500 (Fig. 2). The ICC between the LH500 and NBM-200 was 0.69, while that between the LH500 and the HemoCue was 0.86 (Table 1). A Bland-Altman plot showed that the 2 SD difference of Hb measurements between the LH500 and the NBM-200 was >2.0 g/dL, while that between the LH500 and the HemoCue was <2.0 g/dL (Fig. 3).
Comparison of Hb levels in the group of participants who were deemed ineligible to donate blood showed that the average Hb measurement obtained using the NBM-200 was 12.4 g/dL, while that obtained using the HemoCue was 12.2 g/dL (Table 1). The average Hb was 12.8 g/dL using the NBM-200 and 12.9 g/dL using the HemoCue in the group of participants in whom the Hb values were 12.0-12.4 g/dL by the LH500 (those who were eligible to donate plasma or platelets but not whole blood).
Of the 70 donors who were deemed ineligible to donate blood by the LH500 (i.e., they had a Hb <12.5 g/dL), 43 donors (61.4%) were deemed eligible to donate whole blood by the NBM-200, while 39 donors (55.7%) were deemed eligible to donate whole blood by the HemoCue (Table 2). Among the male donors who were deemed ineligible to donate blood by the LH500, 3 and 2 male donors were deemed eligible to donate by the NBM-200 and the HemoCue, respectively. Among female donors, 40 and 39 female donors were deemed eligible to donate by the NBM-200 and the HemoCue, respectively. The numbers of donors who were eligible to donate whole blood among the donors who were initially deemed ineligible to donate any kind of blood were 23 and 16 by NBM-200 and HemoCue, respectively.
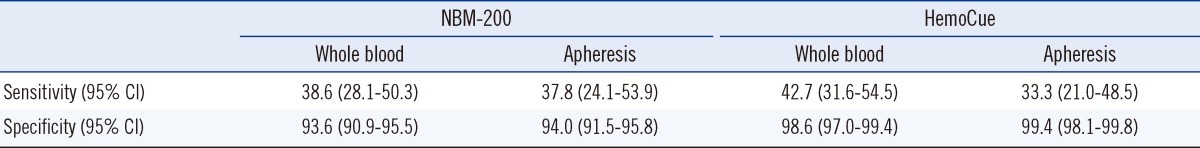
The sensitivities of the NBM-200 and HemoCue to ineligible donors were 33.3-42.7%, while their specificities to eligible donors were 93.6-99.4% (Table 3).
In this study, we attempted to assess Hb estimation accuracy using a noninvasive Hb sensor and the currently used POCT device, the HemoCue, to test for anemia during the blood donor screening process.
The Hb measurements obtained using the NBM-200 showed a left-skewed distribution. The Hb measurements obtained using the NBM-200 also tended to be greater than those obtained using the LH500, which could be a threat to protection of donors' health. The findings imply that use of the NBM-200 as a pre-donation screening tool should occur only with meticulous caution.
Sensitivity and specificity analyses showed that the NBM-200 and HemoCue failed to detect more than half of the ineligible donors but allowed most eligible donors to donate blood.
Analyses among subgroups showed that the subgroup ICCs were lower than the total group ICCs and that the smaller number of participants was attributed to the lower ICCs. The ICCs by the NBM-200 with LH500 implied substantial agreement, while those by the HemoCue with LH500 implied almost perfect agreement. The ICCs by the NBM-200 were lower than those by the HemoCue in all subgroups.
Analysis according to gender showed that female donors who were not eligible to donate blood were at greater risk of being falsely identified as eligible, which can create a substantial donor safety risk. As such, the use of these POCT devices in the pre-donation screening process should occur meticulously with adequate measures (e.g., adjustment of Hb criteria level) to guarantee donor safety.
Many researchers have reported no differences in the Hb measurements obtained using noninvasive Hb sensors versus automated hematology analyzers [8, 9]. However, our study showed only moderate agreement between Hb measurements obtained using the NBM-200 and those obtained using the LH500. Since Hb measurement inaccuracy might endanger a donor's health, strict regulatory compliance should be used in the selection of pre-donation screening tools. In general, "strong agreement" is not adequate to ensure donors' health since accurate Hb estimation in blood donors is crucial to the prevention of adverse blood donation reactions and the exclusion of ineligible blood donors.
The noninvasive Hb sensor mentioned here does not require venipuncture, is not affected by sampling error, and does not require the availability of highly skilled personnel. The measuring system of the noninvasive Hb sensor comprises electronic circuitry, embedded software, and an attached finger sensor probe, and it automatically and continuously performs a self-test and calibration check during measurement sessions. The noninvasive Hb sensor uses a validation process different from the conventional quality control materials of the automated hematology analyzer and the control cuvette of the HemoCue.
Our result showed that the average Hb values obtained by the HemoCue hemoglobinometer were 0.3 g/dL higher than the actual Hb value. Similar results have been reported by other investigators [10-14]. However, the ICCs between the HemoCue and the LH500 showed almost perfect agreement in this study.
The HemoCue can use capillary or venous blood in its Hb estimations. The Hb measurements of venous blood are reliable, but the accuracy and precision of capillary blood measurements are very much dependent on the technical skills of the person performing the procedure. The false deferral rate by the HemoCue seems to be due to a lack of reliability when this method is used on capillary blood samples.
Many investigators have reported that capillary Hb values are higher than venous Hb values [13, 15]. Since the donor acceptance criteria described above were established using venous Hb measurements, the difference between capillary and venous hematological results must be kept in mind.
The use of capillary samples for the diagnosis of anemia has been claimed to be inappropriate [16]. Capillary Hb is affected by the drop used (first, second, third, or fourth), exposure of cuvettes to moisture, and air bubbles that may occur within a cuvette [17]. Poor peripheral circulation, lack of cooperation, and instantaneous sedimentation of red cells in large drops of blood due to slow processing may all be associated with Hb measurement inaccuracy in the use of capillary blood [18]. Another report showed that pooling and mixing drops of skin puncture blood prior to analysis improved the Hb measurement precision of the HemoCue, but this is not feasible as a POCT device when using capillary blood [19]. If the Hb estimation results are higher than the actual values, a person with low Hb might be allowed to donate blood, which could both adversely affect the donor and hinder blood quality.
In the current study, we tried to minimize the sampling bias by allowing only trained staff to conduct the study and by limiting the number of blood collection sites to 2. Despite these efforts, the sampling bias may not have been completely eliminated. Since the subjects were blood donor volunteers, the range of Hb values was not wide enough to make an accurate assessment. In addition, the Hb measurements of most of the volunteers were higher that the donor eligibility criteria. The ability to differentiate eligible and ineligible donors is critical in protecting donor health. The number of study subjects who were not eligible to make a donation was too small to show the obvious Hb estimation differences between the NBM-200 and the HemoCue.
In summary, the results of this study imply that, if used very carefully, the NBM-200 can differentiate eligible and ineligible donors. Even though the NBM-200 eliminates the need for an invasive finger prick or venous blood draw, its use in donor pre-screening should occur with meticulous attention. Hb measurements by the HemoCue showed excellent agreement with those by the automated hematology analyzer. Since pre-donation testing is crucial to protecting donors' health, complete evaluation of an instrument should be performed prior to its use. Every effort should be made to improve the quality control of Hb measurements to ensure donor safety and guarantee good blood quality.
Acknowledgements
The authors are grateful to all volunteers who participated in our study. We also acknowledge all staff members of Hanmaum Blood Center. We acknowledge statistical support provided by Dr. Jieun Kim and Min Wong Kang of Biostatistics Collaboration Unit at Yonsei University College of Medicine.
References
1. Cable RG. Hemoglobin determination in blood donors. Transfus Med Rev. 1995; 9:131–144. PMID: 7795331.

2. Boulton FE, Nightingale MJ, Reynolds W. Improved strategy for screening prospective blood donors for anaemia. Transfus Med. 1994; 4:221–225. PMID: 7820230.

3. James V, Jones KF, Turner EM, Sokol RJ. Statistical analysis of inappropriate results from current Hb screening methods for blood donors. Transfusion. 2003; 43:400–404. PMID: 12675728.

4. Ross DG, Gilfillan AC, Houston DE, Heaton WA. Evaluation of hemoglobin screening methods in prospective blood donors. Vox Sang. 1986; 50:78–80. PMID: 3962283.

5. Noiri E, Kobayashi N, Takamura Y, Iijima T, Takagi T, Doi K, et al. Pulse total-hemoglobinometer provides accurate noninvasive monitoring. Crit Care Med. 2005; 33:2831–2835. PMID: 16352948.

6. Neville RG. Evaluation of portable haemoglobinometer in general practice. Br Med J (Clin Res Ed). 1987; 294:1263–1265.

7. Lotfi R, Wernet D, Starke U, Northoff H, Cassens U. A noninvasive strategy for screening prospective blood donors for anemia. Transfusion. 2005; 45:1585–1592. PMID: 16181209.

8. Gayat E, Aulagnier J, Matthieu E, Boisson M, Fischler M. Non-invasive measurement of hemoglobin: assessment of two different point-of-care technologies. PLoS One. 2012; 7:e30065. PMID: 22238693.

9. Landis JR. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174. PMID: 843571.

10. Dhingra-Kumar N. CuSO4 gravimetric method for Hb screening of prospective donors--should it be discarded? Transfus Med. 1997; 7:245–247. PMID: 9316227.
11. Sawant RB, Bharucha ZS, Rajadhyaksha SB. Evaluation of hemoglobin of blood donors deferred by the copper sulphate method for hemoglobin estimation. Transfus Apher Sci. 2007; 36:143–148. PMID: 17382593.

12. Bhaskaram P, Balakrishna N, Radhakrishna KV, Krishnaswamy K. Validation of hemoglobin estimation using Hemocue. Indian J Pediatr. 2003; 70:25–28. PMID: 12619948.

13. Neufeld L, García-Guerra A, Sánchez-Francia D, Newton-Sánchez O, Ramírez-Villalobos MD, Rivera-Dommarco J. Hemoglobin measured by Hemocue and a reference method in venous and capillary blood: a validation study. Salud Publica Mex. 2002; 44:219–227. PMID: 12132319.

14. Prakash S, Kapil U, Singh G, Dwivedi SN, Tandon M. Utility of HemoCue in estimation of hemoglobin against standard blood cell counter method. J Assoc Physicians India. 1999; 47:995–997. PMID: 10778695.
15. Gordeuk VR, Brittenham GM, Bravo J, Hughes MA, Keating LJ. Prevention of iron deficiency with carbonyl iron in female blood donors. Transfusion. 1990; 30:239–245. PMID: 2180144.

16. Cogswell ME, Parvanta I, Yip R, Brittenham GM. Innaccuracy of capillary hemoglobin testing for the detection of anemia and iron deficiency during pregnancy. Am J Epidemiol. 1998; 147(S5):206.
17. Bahadur S, Jain S, Jain M. Estimation of hemoglobin in blood donors: A comparative study using hemocue and cell counter. Transfus Apher Sci. 2010; 43:155–157. PMID: 20667788.

18. Conway AM, Hinchliffe RF, Earland J, Anderson LM. Measurement of haemoglobin using single drops of skin puncture blood: is precision acceptable? J Clin Pathol. 1998; 51:248–250. PMID: 9659272.

19. Mills AF. Screening for anaemia: evaluation of a haemoglobinometer. Arch Dis Child. 1989; 64:1468–1471. PMID: 2817932.

Fig. 1
Histograms of the hemoglobin levels measured using the LH500 (A), the NBM-200 (B), and the HemoCue (C).
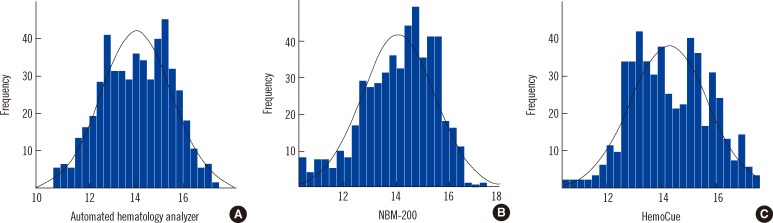
Fig. 2
Scatter plots of the hemoglobin levels measured using the LH500 vs. the NBM-200 (A) and the hemoglobin levels measured using the LH500 vs. the HemoCue (B).
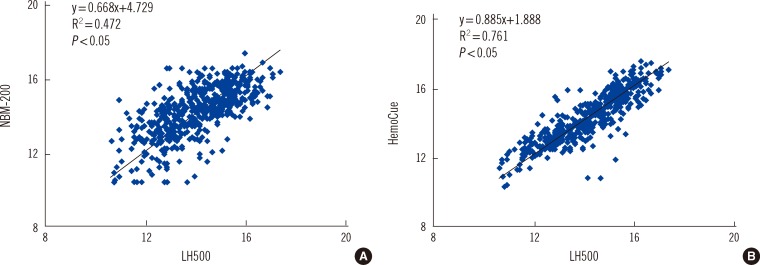
Fig. 3
Bland-Altman plot of the difference between the LH500 and the 2 point-of-care testing devices (A, NBM-200; B, HemoCue). Abbreviation: AHA, automated hematology analzyer.
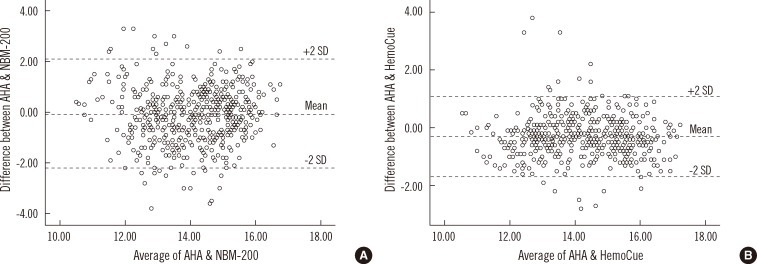
Table 1
Hemoglobin measurements and intraclass correlation coefficient (ICC) of subgroups according to whole blood donation and apheresis eligibility




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download