Abstract
Background
A reliable rapid assay for hepatitis C virus (HCV) may be helpful in various clinical settings. We evaluated the performance of the OraQuick HCV Rapid Antibody Test (OraSure Technologies Inc., Bethlehem, PA, USA).
Methods
Clinical sensitivity and specificity were evaluated with oral fluids and sera from 137 patients diagnosed with hepatitis C and 300 healthy blood donors in a multi-center collaborative study. The stored sera of 200 proven HCV-infected patients and 200 healthy subjects were also evaluated. Analytical sensitivity was estimated with 4 commercial seroconversion panels and 7 Korean reference panels. The performance of 4 laboratory-based tests (3 chemiluminescence assays and 1 enzyme immunoassay) and 4 rapid test kits was compared. We also assessed the interference due to bilirubin, hemoglobin, lipid, rheumatoid factor, multipara, and several viral infections.
Results
The clinical sensitivity and specificity of the OraQuick HCV test using oral fluid were 97.8% (95% confidence interval [CI], 93.2-99.4%) and 100% (95% CI, 98.4-100%), respectively. The clinical sensitivity using serum samples was 100%. Using the 4 seroconversion panels, the OraQuick HCV test showed results comparable to those of the laboratory-based assays; its analytical sensitivity was higher than that of the other rapid test kits. There was no cross-reactivity with common interfering factors.
Hepatitis C is a chronic disease affecting approximately 130-170 million people worldwide. According to the WHO, annually, more than 350,000 people die from hepatitis C-related liver diseases and 3-4 million people are infected with hepatitis C virus (HCV) [1]. Approximately 3% of the world's population is estimated to be infected with HCV. The prevalence of HCV in Korea is estimated to be 1.12-1.48% in the middle-aged population [2]. The Korea Centers for Disease Control and Prevention is focusing on the increasing trend of hepatitis C and has included this disease in the Korea National Health and Nutrition Survey [3]. More than 75% of HCV-infected individuals develop chronic liver diseases, 20-30% develop cirrhosis after 20-30 yr, and 1-4% die from cirrhosis or liver cancer. HCV infection is curable with effective antiviral agents. However, since no vaccine is currently available, early diagnosis and intervention are very important to prevent disease progression.
A simple, non-instrumented, rapid, point-of-care test, the OraQuick HCV Rapid Antibody Test (OraSure Technologies Inc., Bethlehem, PA, USA), was recently developed to identify HCV infection and approved by the US Food and Drug Administration (FDA) for use with venous whole blood (June 2010) and fingerstick blood (February 2011). Although it was approved for use with venous and fingerstick blood only in the US, it can detect anti-HCV antibodies from oral fluid specimens and is reported to be suitable for aiding the diagnosis of HCV infection [4]. This collaborative study aimed to evaluate the clinical sensitivity and specificity of this test, compare the performance of oral fluid tests and serum tests, and validate the absence of interference of common interfering substances.
The OraQuick HCV Rapid Antibody Test utilizes an indirect lateral flow immunoassay to detect antibodies against the recombinant core as well as NS3 and NS4 antigens with synthetic HCV peptides. Oral fluid samples were collected by swiping the gums with the collection pad of the device, and serum was collected using a specimen loop and mixed in the developer solution before inserting the device into the vial. The test procedures were performed according to the manufacturer's guidelines. The results were interpreted after 20 to 40 min, and the validity of each assay was confirmed by a built-in procedural control and external quality controls.
For the comparison study, Architect (Abbott Laboratories, Abbott Park, IL, USA), AxSYM (Abbott Laboratories), E170 (Roche Diagnostics Limited, Rotkreuz, Switzerland), ADVIA Centaur XP (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA), and Elecsys Systems (Roche Diagnostics Limited) as well as 3 rapid test kits for anti-HCV antibody-Asan Easy Test HCV (Asan Pharmaceutical, Seoul, Korea), SD BIOLINE HCV (SD, Yongin, Korea), and Genedia HCV Rapid LF (Green Cross Medical Science, Yongin, Korea)-were used.
A total of 137 patients previously diagnosed with hepatitis C on the basis of clinical and laboratory tests from 2 different hospitals (Samsung Medical Center and Seoul National University Bundang Hospital) were enrolled from March 2011 to May 2012 for the evaluation of clinical sensitivity. The patients had various histories and were diagnosed from 19 yr before the study to very recently. Patients who underwent antiviral treatment were not excluded. Oral fluid samples were collected and promptly tested at the outpatient clinic. After oral fluid testing, venous blood was drawn from each patient and centrifuged at 1,500 rpm for 10 min, and the separated serum was stored at -80℃ until testing. However, sera could be obtained in only 114 patients who were enrolled in the oral fluid test. Sera were tested with OraQuick HCV and SD BIOLINE HCV. The time interval between serum collection and testing was within 1 month. If the oral fluid test result was non-reactive, the OraQuick HCV with oral fluid was repeated and the serum anti-HCV test by Architect was performed. The purpose of repeated oral fluid test was to confirm whether the results were false negatives. All procured sera were tested by OraQuick HCV and SD BIOLINE HCV. OraQuick HCV test results were evaluated with another set of serum samples collected from 200 HCV-infected patients previously proven to be positive for HCV RNA and stored in the Human Serum Bank (Chung-Ang University). The test was repeated if the OraQuick HCV result was negative.
To evaluate the clinical specificity of the test, the oral fluids of 300 randomly selected blood donors were tested at the site of blood donation. If the result was reactive, the test was repeated immediately. All the results were compared with those of the serum anti-HCV assay by Architect. If there was a discrepancy between the results, the serum anti-HCV test was repeated and confirmed by Western blotting (HCV BLOT 3.0; MP Biomedicals, Santa Ana, CA, USA) and HCV reverse transcriptase-PCR (COBAS AmpliPrep/COBAS TaqMan HCV Test v2.0; Roche Diagnostics Limited), if necessary. Furthermore, an additional 200 negative serum specimens provided by the Human Serum Bank were tested with OraQuick HCV.
All testing was carried out after receiving informed consent, according to the protocols approved by the institutional review board of each institution.
Analytical sensitivity was evaluated by comparing the test assays to other assays using seroconversion panels (SeraCare Life Sciences, Milford, MA, USA) as well as HCV reference panels purchased from the Korea Food and Drug Administration (KFDA).
Four kinds of seroconversion panels including HCV genotypes 1a, 2b, and 3a were tested with OraQuick HCV; the results were compared with those from 4 laboratory-based anti-HCV tests (AxSYM, Architect, Centaur, and E170) as well as 3 rapid tests (Asan, SD, and Green Cross). The first bleed days detected by each method as well as the assigned results designated in the manufacturer's inserts were compared.
Seven different HCV reference panels provided by the KFDA (Table S1 available as supplemental data at ALM online) were diluted with the normal serum and tested with the 4 laboratory-based anti-HCV tests and 3 rapid tests. Because the antibody titers vary, each panel was diluted to make 25 different dilutions to compare the assay performance according to the signal-to-cutoff (S/C) values of the laboratory-based assays. First, the index dilution that showed an S/C value of 3-5 by Architect was determined. Then, higher-titer dilutions were made by adding 2 µL undiluted panel sample cumulatively from the index dilution. Lower-titer dilutions were made by serial dilution from the index dilution (Table S2 available as supplemental data at ALM online).
Each dilution was tested with the 4 rapid test kits, and the S/C values of the Architect, Centaur, AxSYM, and E170 tests with the maximal dilution that can be detected by each rapid test were found and averaged. The Wilcoxon rank sum test was performed to determine statistical significance.
Common potentially interfering factors in immunoassays, such as bilirubin, triglycerides, and hemoglobin, were studied by adding these interfering substances to 30 negative and 30 positive specimens. The test results were compared before and after the addition of the interfering substances. These included specimens with hyperbilirubinemia (10.3-41.4 mg/dL), hemolysis (0.5-2.6 g/dL hemoglobin), and hypertriglyceridemia (100-1,433 mg/dL). Twenty specimens with rheumatoid factor, 20 specimens with other viral infections (i.e., HIV, or hepatitis A or B virus), and 20 specimens from multipara were also analyzed in the same manner for the interference and cross-reactivity study.
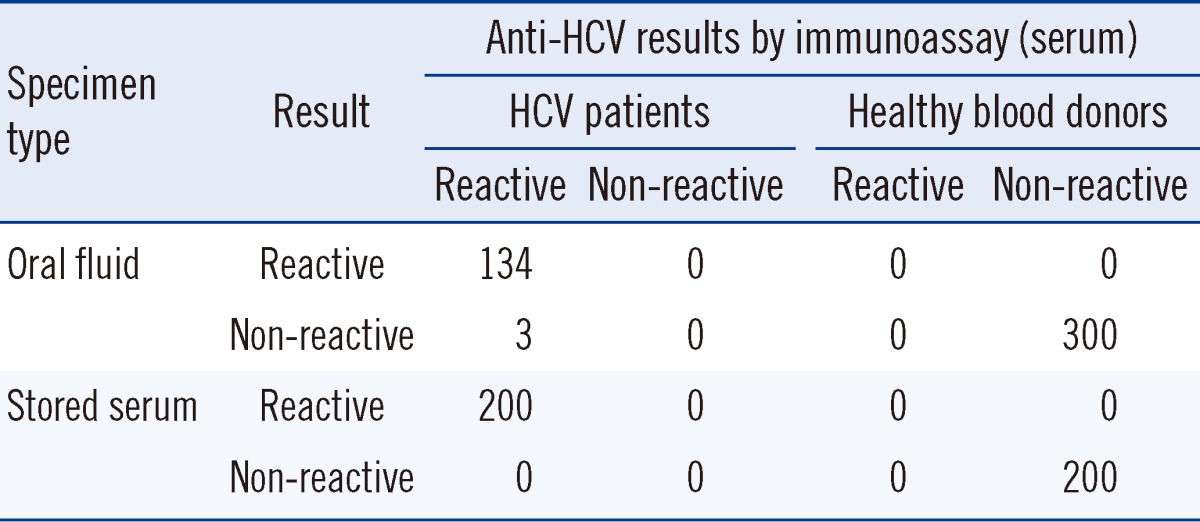
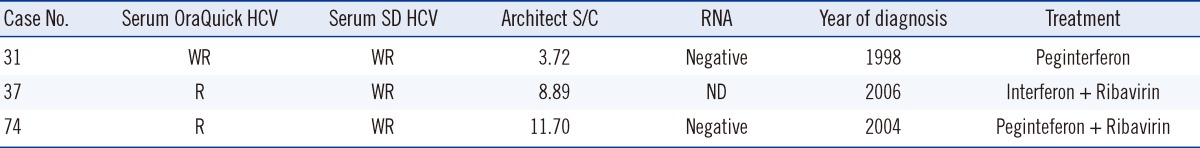
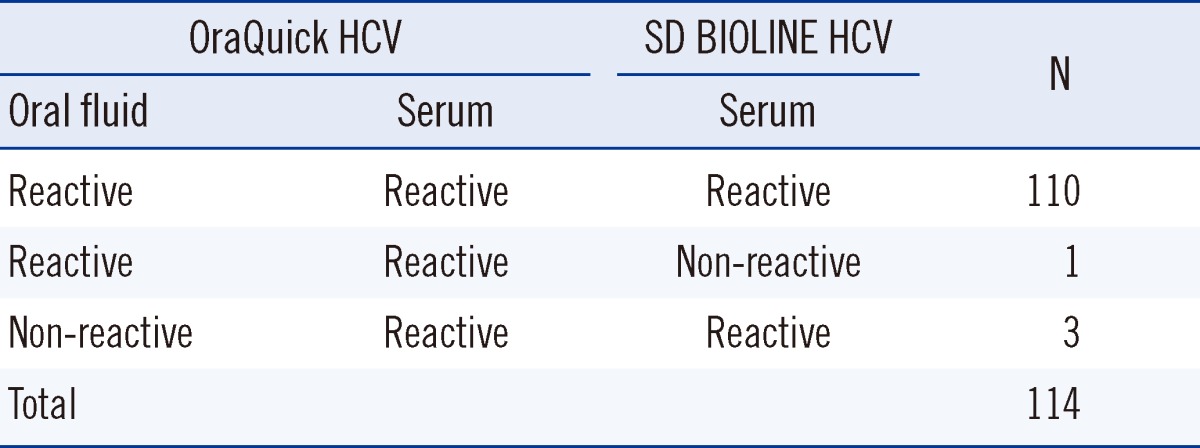
Among the 137 patients diagnosed with HCV, 134 were reactive according to the oral fluid OraQuick HCV test, resulting in a clinical sensitivity of 97.8% (95% confidence interval [CI], 93.2-99.4%), while none were reactive among the 300 healthy blood donors, resulting in 100% specificity (95% CI, 98.4-100%) (Table 1). The 3 patients who showed false-negative results according to the oral fluid test had antibody titers with a S/C value of 3-12 with Architect; had been suffering from the disease for 5, 7, and 13 yr, respectively; and received anti-viral treatment. Therefore, RNA was not detected in 2 of them (Table 2). Additionally, all the 200 proven HCV-positive sera samples showed reactivity with the OraQuick HCV test. In the serum tests of the 114 patients enrolled in the oral fluid test, all the sera samples including those of the 3 non-reactive patients in the oral fluid test were reactive with OraQuick HCV and all, but 1 case, were reactive with the SD BIOLINE HCV test (Table 3). Therefore, the OraQuick HCV showed 100% clinical sensitivity (95% CI, 97.7-100%) when serum was used as a specimen in the patient group. There were no invalid tests during the trial.
The OraQuick HCV test showed results comparable to those of the 4 current laboratory-based anti-HCV tests for all the seroconversion panels tested (Table S3 available as supplemental data at ALM online). Interestingly, all the rapid kits seemed to be more sensitive than 2 of the laboratory-based assays with the genotype 3a panel (PHV921), in which the rapid tests detected anti-HCV antibody 10 days earlier than Architect and E170 did. The assay performance of the OraQuick HCV test with seroconversion panels showed sensitivity comparable to that of 2 KFDA-approved rapid tests. However, 1 of the 3 KFDA-approved rapid tests could not detect certain conversion panels at all (Table S4 available as supplemental data at ALM online).
Analytical sensitivity evaluation with the KFDA reference panels showed that the OraQuick HCV test was quite comparable to existing laboratory-based anti-HCV assays such as Architect, Centaur, and AxSYM (Table S5 available as supplemental data at ALM online). According to the S/C values of the laboratory-based anti-HCV tests, which represent the maximum dilution for which a rapid test kit's result is reactive, the rapid kits showed comparable sensitivity except the Green Cross kit-the results of which were the least sensitive and most unstable (Fig. 1 and Table S6 available as supplemental data at ALM online).
Neither bilirubin (up to 10 mg/dL) and hemoglobin (0.5 g/dL) nor triglycerides (up to 300 mg/dL) showed any interference with the OraQuick HCV test results. Furthermore, there was no cross-reactivity with sera positive for rheumatoid factor, multipara, other viral infections, such as HIV, or hepatitis A and B.
Rapid tests are relatively easy to develop and there are many products in the market. Because rapid assays are even being used as blood-screening tools in developing countries, the International Consortium for Blood Safety (ICBS) conducted a study to evaluate the sensitivity and specificity of rapid assays and found that most methods have lower sensitivity than those of conventional laboratory-based assays [4].
Nevertheless, rapid assays have some advantages. They are easy to perform, do not require expensive equipment or experienced personnel, and the results can be obtained immediately. They have the potential to provide better accessibility and flexibility in various clinical settings.
The rapid test using oral fluid has additional advantages: it does not require venipuncture or fingerstick. Thus, this increases the compliance of individuals who want to be tested and protects medical personnel from undesirable biohazards. Therefore, the rapid assay using oral fluid as a specimen is ideal if its clinical performance is acceptable.
The main drawbacks of rapid assays are relatively inferior clinical sensitivity and the operator's subjectivity in the interpretation of the test results. The OraQuick HCV test seems to overcome these drawbacks to some extent. The OraQuick HCV test is reported to have the greatest degree of inter-operator agreement in result interpretation [5]. Furthermore, we did not encounter any ambiguous results during our study, even when the operators were not experienced laboratory technicians. Moreover, only a small quantity of specimen (5 µL) is required.
The present study combined laboratory-based and field studies. The results show that the clinical sensitivity and specificity of the OraQuick HCV test are comparable to those of the traditional laboratory-based immunoassays in both studies. In patients diagnosed with HCV, the OraQuick HCV test showed 97.8% clinical sensitivity with oral fluids and 100% with sera. The observed sensitivity was slightly lower than 99.2% (95% CI, 95.5-100%), which was reported previously [6]. The clinical specificity was 100%. The performance of field studies are generally inferior to that of laboratory-based studies because of the degree of specimen characterization, differences in the technical expertise of operators, and other variables. The sensitivity of the rapid HCV test in field studies is reported to range from 88.3% to 99.3% with serum [5] and 81.8% to 94.7% with oral fluid among injection drug abusers [7, 8].
The major limitation of this study is that all the enrolled patients were clinically proven HCV patients, most of who were under or completed antiviral treatment. To evaluate rapid assays for clinical diagnosis, it is desirable to enroll fresh patients including those in the early phase of the disease. However, the results show that the assay can detect anti-HCV antibodies in patients who have undergone antiviral treatment. The HIV antibody test is known to be less sensitive in patients undergoing antiviral therapy [9]. Anti-HCV antibody is known to decrease after sustained virological response, and approximately 1.2% of the patients can be even seroconverted [10]. The 3 cases that exhibited false-negative results with oral fluid showed the presence of anti-HCV antibodies in serum, and the patients had been suffering from the disease for a long time. Because the serum rapid tests detected the antibodies, the false-negative results may be due to lower antibody titer in oral fluid or some unknown interfering factors. Therefore, at present, it is recommended to use the OraQuick HCV test with serum if a serum sample is available. Although our study demonstrated relatively high clinical sensitivity, further studies with patients in the early phase of infection are required to confirm the clinical utility of the test, especially for blood screening. Furthermore, the influence of HIV infection on the assay must be clarified, because HIV infection can induce false-negative results in the anti-HCV assay [5].
A screening test for a disease with a low prevalence should have high specificity. In this regard, the high specificity of the OraQuick HCV test is promising, because it does not exhibit any interference due to commonly encountered conditions such as hemoglobin, turbidity, autoantibodies, or other viral illnesses.
We compared its analytical sensitivity with that of commonly used laboratory-based assays and rapid assays using multiple seroconversion panels and Korean panels. The results show that the OraQuick HCV test is comparable to current conventional laboratory-based assays and more consistent than other rapid test kits evaluated according to the S/C values of 3 different automated conventional test results corresponding to the detection limit of each rapid assay.
HCV exhibits a different genotype distribution with respect to geographic location. In addition, the antiviral antibody concentration in humoral fluids can vary among ethnic groups. Therefore, the performance of rapid test kits should be evaluated using clinical specimens from the country that the study is being performed in. In Korea, most clinical isolates are of genotype 1b or 2a. In this study, there were slight differences in the analytical sensitivity between genotypes. However, it is difficult to conclude with a reason for this, because it is reported that the test performances of rapid assays is not related to the genotype [4].
At present, various healthcare-related procedures, especially in nonhospital healthcare settings, are suspected to be the major modes of acquisition in the general population other than high-risk groups such as intravenous drug abusers [11, 12]. In the US, approximately 45% of infected persons do not have any known exposure risk [13]. Potential risk factors may include chronic hemodialysis [14], vertical transmission, intranasal drug use, tattoos and piercings, and invasive medical procedures. This warrants the development of a public policy to prevent such community-acquired HCV infections in healthcare facilities. A reliable and rapid screening test appears to be an essential component in such a strategy. The OraQuick HCV test is reported to be a useful tool in such situations [6, 15]. We expect that the rapid HCV test will be widely used to various medical and non-medical environments in the future.
Acknowledgement
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A100054).
References
1. Hepatitis C. Fact sheet No. 164. World Health Organization. http://www.who.int/mediacentre/factsheets/fs164/en/index.html (Update on Jun 2011).
2. Shin HR. Epidemiology of hepatitis C virus in Korea. Intervirology. 2006; 49:18–22. PMID: 16166784.

3. Kim SJ. Viral hepatitis surveillance system and statue of C hepatitis sentinel surveillance in Korea. Public Health Wkly Rep. 2012; 5:214–219.
4. Scheiblauer H, El-Nageh M, Nick S, Fields H, Prince A, Diaz S. Evaluation of the performance of 44 assays used in countries with limited resources for the detection of antibodies to hepatitis C virus. Transfusion. 2006; 46:708–718. PMID: 16686838.

5. Smith BD, Drobeniuc J, Jewett A, Branson BM, Garfein RS, Teshale E, et al. Evaluation of three rapid screening assays for detection of antibodies to hepatitis C virus. J Infect Dis. 2011; 204:825–831. PMID: 21849279.

6. Lee SR, Kardos KW, Schiff E, Berne CA, Mounzer K, Banks AT, et al. Evaluation of a new, rapid test for detecting HCV infection, suitable for use with blood or oral fluid. J Virol Methods. 2011; 172:27–31. PMID: 21182871.

7. Smith BD, Teshale E, Jewett A, Weinbaum CM, Neaigus A, Hagan H, et al. Performance of premarket rapid hepatitis C virus antibody assays in 4 national human immunodeficiency virus behavioral surveillance system sites. Clin Infect Dis. 2011; 53:780–786. PMID: 21921221.

8. Jewett A, Smith BD, Garfein RS, Cuevas-Mota J, Teshale EH, Weinbaum CM. Field-based performance of three pre-market rapid hepatitis C virus antibody assays in STAHR (Study to Assess Hepatitis C Risk) among young adults who inject drugs in San Diego, CA. J Clin Virol. 2012; 54:213–217. PMID: 22560051.

9. O'Connell RJ, Merritt TM, Malia JA, VanCott TC, Dolan MJ, Zahwa H, et al. Performance of the OraQuick rapid antibody test for diagnosis of human immunodeficiency virus type 1 infection in patients with various levels of exposure to highly active antiretroviral therapy. J Clin Microbiol. 2003; 41:2153–2155. PMID: 12734265.
10. Kee KM, Wang JH, Hung CH, Chen CH, Lee CM, Chang KC, et al. Decreased anti-hepatitis C virus titer and associated factors in chronic hepatitis C patients after sustained virological response: a prospective study. J Gastroenterol Hepatol. 2012; 27:1106–1111. PMID: 22004331.

11. Thompson ND, Perz JF, Moorman AC, Holmberg SD. Nonhospital health care-associated hepatitis B and C virus transmission: United States, 1998-2008. Ann Intern Med. 2009; 150:33–39. PMID: 19124818.

12. Kim JY, Won JE, Jeong SH, Park SJ, Hwang SG, Kang SK, et al. Acute hepatitis C in Korea: different modes of infection, high rate of spontaneous recovery, and low rate of seroconversion. J Med Virol. 2011; 83:1195–1202. PMID: 21567423.

13. Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012; 61:1–32. PMID: 22895429.
14. Hepatitis C virus transmission at an outpatient hemodialysis unit--New York, 2001-2008. MMWR Morb Mortal Wkly Rep. 2009; 58:189–194. PMID: 19265779.
15. Lee SR, Yearwood GD, Guillon GB, Kurtz LA, Fischl M, Friel T, et al. Evaluation of a rapid, point-of-care test device for the diagnosis of hepatitis C infection. J Clin Virol. 2010; 48:15–17. PMID: 20362493.

Fig. 1
Comparison of reactive zones of the Architect and 4 rapid test kits according to the E170 S/C value and dilution. The shaded areas represent the reactive zones of each test method.
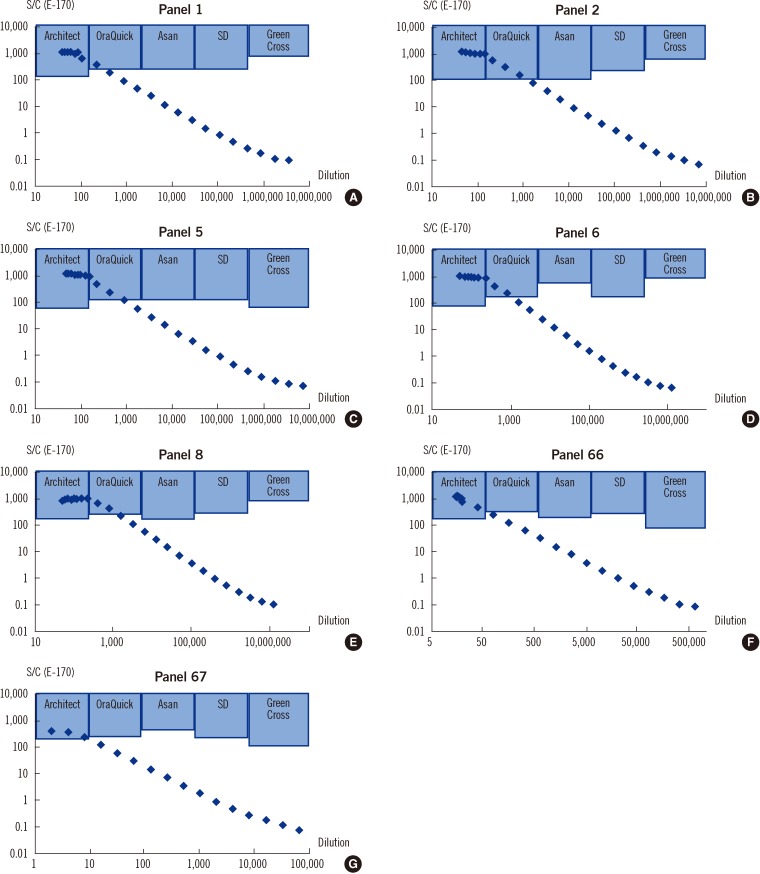
Table 1
Summary of the clinical sensitivity and specificity study of the OraQuick HCV Rapid Antibody Test with oral fluid and serum specimens




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download