INTRODUCTION
HLA-B27 typing is widely practiced as an aid for diagnosing spondyloarthropathies [
1]. Many HLA-B27 typing methods are in use, and several DNA-based methods are increasingly being used in clinical laboratories [
2]. However, flow cytometry (FC) using monoclonal antibodies (FC HLA-B27 typing) is still the most extensively used method, because it is economical and relatively simple [
3].
However, it has been recommended that FC HLA-B27 typing be performed using blood samples within 24 hr of venipuncture [
4], and therefore, "batch testing" of stored samples is not possible. Routine batch testing is usually performed on a set day of the week by using stored blood samples collected during the previous week. Furthermore, the results of HLA-B27 typing are not usually reported immediately, because they are required for "next" patient visits. However, clinical laboratories serving low-volume hospitals inevitably face inefficiencies in terms of time, cost, and labor because of the small numbers of samples collected in order to comply with the principle of testing "fresh" blood samples. Whole blood can be stored after being treated with commercially available white blood cell (WBC) stabilization solutions such as TransFix (Cytomark, Buckingham, UK) or Cyto-Chex Reagent (Strek Laboratories, Omaha, NE, USA); however, these reagents were developed for control materials [
5], and are not intended for nor have been evaluated using patient samples.
According to the guidelines issued by the European Federation for Immunogenetics (EFI) [
6] and the American Society for Histocompatibility and Immunogenetics (ASHI) [
7], cellular controls should be run as a part of each FC HLA-B27 typing batch to verify reagent specificity [
5,
8]. Fresh blood samples with known HLA-B27 typing results would be ideal, but are logistically difficult to obtain. Cryopreserved and thawed mononuclear cell suspensions with known results can be used, if each laboratory validates that thawed cells exhibit reactivity patterns similar to those of the same cells when tested freshly [
9]. A few manufacturers provide HLA-B27+ control cells, which are stabilized preparations of human cell lines. However, if the prolonged storage of patient blood samples is allowed, FC HLA-B27 typing can be performed in a batch manner, and in-house cellular controls could easily be prepared from known patient samples.
One of the commercial monoclonal antibody reagents for FC HLA-B27 typing, the IOTest HLA-B27-FITC/HLA-B7-PE (Beckman Coulter, Miami, FL, USA) consists of two kinds of monoclonal antibodies: ABC-m3 directed toward HLA-B27/B2708 antigens and BB7.1 directed toward HLA-B7 antigens [
4]. The former is a fluorescein-5-isothiocyanate (FITC)-conjugated antibody that targets HLA-B27, but is also weakly reactive to the HLA-B7 antigens, while the latter is a phycoerythrin (PE)-conjugated antibody that targets HLA-B7 and blocks the cross-reactivity of the former to the HLA-B7 antigens [
10].
Batch testing using stored blood samples for FC HLA-B27 typing demands that the antigenicities of HLA Class I antigens on stored cells remain detectable to allow differentiation within the B7 cross-reactive group (CREG), which includes the HLA-B7, 13, 27, 2708, 37, 41, 42, 47, 48, 54, 55, 56, 60, 61, 73, and 81 antigens [
11]. In contrast, platelets carry HLA Class I antigens and not HLA Class II antigens [
12]. Furthermore, the cryopreservation of platelets is relatively straightforward because of their simple anuclear structures [
13], and therefore, we supposed that platelets might be adequate for FC typing after cryopreservation.
Little data are available regarding the reliability of FC HLA-B27 typing using stored samples. In this study, we sought to determine which of the four methods described above is best for storing patient blood samples without using a commercial preserving solution; three of these methods involved the analysis of lymphocytes (whole blood stored at room temperature, frozen mononuclear cells, and frozen WBCs after lysing red blood cells [RBCs]) and one involved the use of frozen platelets.
Go to :

METHODS
We compared six FC HLA-B27 typing methods: two "immediate" methods and four "storage-based" methods (
Table 1). We then selected the best storage method and evaluated it using patient samples.
Table 1
Outline of the six different methods (two immediate and four storage) used for FC HLA-B27 typing
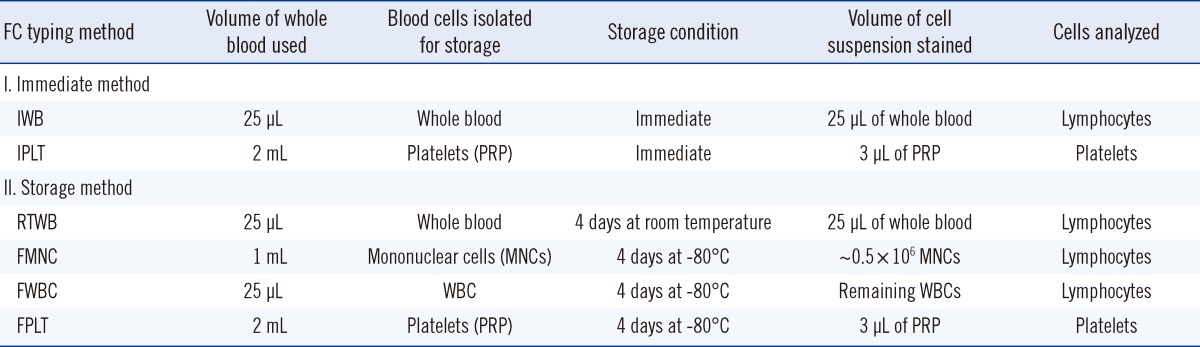

1. Samples
In the present study, we used 164 consecutive EDTA-anticoagulated blood samples sent to the histocompatibility laboratory at Kyungpook National University Hospital during 2011 for DNA-based HLA-A/B/DR or HLA-B27 typing. Of these, 154 samples were obtained from Korean patients and 10 samples were from HLA proficiency surveys (B27A2011 and B27B2011) conducted by the College of American Pathologists. Informed consent was obtained from all patients prior to the study.
2. FC HLA-B27 typing
Each FC typing method shared identical final steps, namely, fluorescent staining, data acquisition, and data analysis, as described later. One IgG2a-FITC/IgG1-PE isotype control (Beckman Coulter) and one negative control were always run simultaneously with the test samples. Cells lacking specific and cross-reacting antigens (i.e., having neither HLA-B27 nor the other B7 CREG antigens) were selected as negative controls from known samples. For each FC typing method, we used negative control cells of the same cell type (lymphocytes or platelets) that had been stored under the same conditions as the test samples.
FC typing methods were designated names by using the following credentials: "(storage temperature)-(status of stored cells);" for example, "RTWB" stands for "(Room Temperature)-(Whole Blood)." One "wash" in this study means: 1) 3 mL of phosphate-buffered saline (PBS) was added to a tube; 2) the tube was vortexed and centrifuged; 3) the supernatant was decanted; and 4) the cell pellet was re-suspended in the remaining or additional PBS in its tube.
The outline of the six FC typing methods is provided in
Table 1. IWB is the "standard" method performed according to the manufacturer's recommendations [
4]. The pre-staining assay steps for each FC typing method (numbered one to six) were as follows:
1) Immediate whole blood (IWB)
Twenty-five microliters of whole blood in EDTA was aliquoted into a microcentrifuge tube. After "fluorescent staining" (described later), stained whole blood was mixed with 1 mL of RBC lysing solution (BD Biosciences, San Jose, CA, USA), vortexed, and incubated for 10 min at room temperature in the dark. The mixture was then moved to a 5 mL polystyrene tube, centrifuged for 5 min at 400×g, and the supernatant was decanted. The remaining WBCs were washed once.
2) Immediate platelets (IPLT)
Whole blood collected in a 5 mL EDTA tube was centrifuged for 10 min at 200×g, and the top 3/4 of the supernatant platelet rich plasma (PRP) was moved to another tube [
14]. Three microliters of PRP was then mixed with 22 µL of PBS in a microcentrifuge tube to achieve the same reaction volume as IWB. Platelets in this suspension underwent fluorescent staining, during which one wash was performed with 3 mL of EDTA-PBS (3.36 g EDTA in 1,000 mL PBS) for 10 min at 1,000×g.
3) Room temperature-stored whole blood (RTWB)
Samples were held for four days at room temperature before processing. The remaining processes were as described for IWB.
4) Frozen mononuclear cells (FMNC)
Using the density gradient centrifugation method, mononuclear cells (MNCs) were separated from 1 mL of whole blood and washed (×3) with RPMI. This suspension was then mixed with the same volume of cryosolution (6% hydroxyethyl starch, 4% fetal calf serum, 5% dimethyl sulfoxide in RPMI; final concentration) in ice water [
15], and stored at -80℃. Just prior to testing, frozen MNCs were immediately thawed in thawing solution (2% fetal calf serum in RPMI). The suspension was then vortexed and centrifuged, and supernatants were discarded. Remaining MNCs were distributed in ~0.5×10
6 cells per tube for fluorescent staining.
5) Frozen WBCs after lysis of the RBCs (FWBC)
One milliliter of RBC lysing solution was added to 25 µL of whole blood in a microcentrifuge tube. After vortexing and incubating for 10 min at room temperature, the mixture was immediately frozen at -80℃ [
16]. Just prior to testing, it was briefly (< 5 min) thawed in a 37℃ water bath, and after centrifugation for 5 min at 400×g, the supernatant was decanted. Remaining WBCs were washed once and then fluorescent stained.
6) Frozen platelets (FPLT)
Platelets were frozen as described by Helmerhorst et al. [
13]. Briefly, PRP (obtained as described for IPLT) was frozen directly at -80℃, and just prior to testing, it was thawed at room temperature. The remaining processes were as described for IPLT.
7) Fluorescent staining
Five microliters of IOTest HLA-B27-FITC/HLA-B7-PE reagent was added to each immediate or stored sample prepared at its defined volume in a microcentrifuge tube, and incubated for 20 min at room temperature with gentle agitation in the dark. For IWB, RTWB, and FWBC, RBCs were removed using RBC lysing solution. After one wash, 50 µL of PBS was added to achieve the volume required for data acquisition. Samples were stored at 4℃ in the dark shortly until data acquisition.
8) Data acquisition
FC was performed using a FACSCalibur flow cytometer running CELLQuest pro software (BD Biosciences). The instrument was calibrated daily using CaliBRITE beads and FACSComp software (BD Biosciences). Fluorescence signals were logarithmically amplified using a 4-log decade scale (10
0 to 10
4) (
Fig. 1).
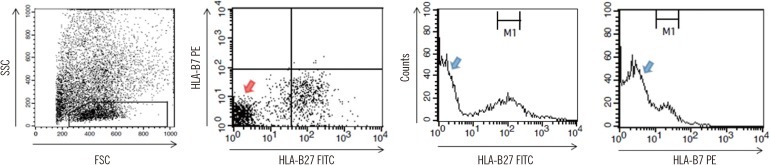
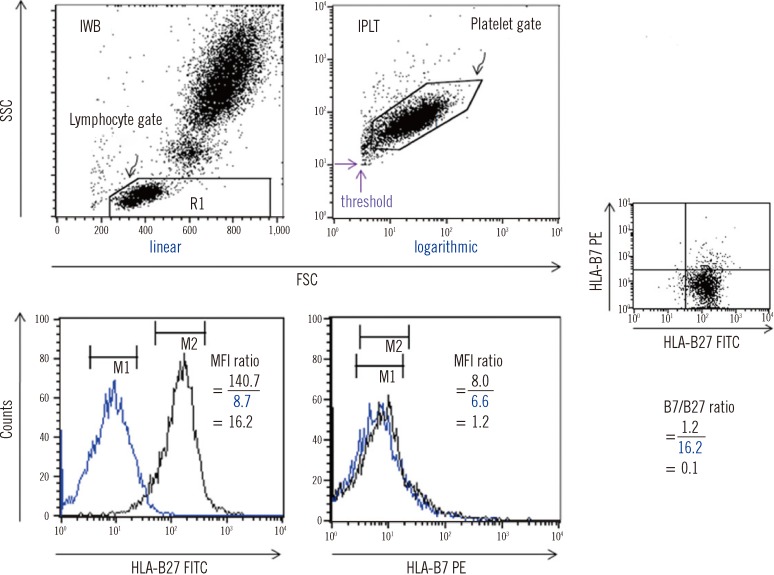 | Fig. 1
FC HLA-B27 typing using the two immediate methods. The black and blue peaks denote a test sample (HLA-B27+) and a negative control, respectively. On each FSC vs. SSC plot, a lymphocyte gate was drawn for IWB using linear amplification, and a platelet gate was drawn for IPLT using logarithmic amplification. For IPLT, the FSC and SSC threshold values (the two purple arrows) were set in order to effectively collect the platelet signals by eliminating unwanted events. MFI values were obtained as the geometric means of the peaks on the HLA-B27 FITC or B7 PE histogram of the gated lymphocytes or platelets. An example of the B27 and B7 MFI ratios obtained by analyzing lymphocytes and the corresponding B7/B27 ratio is presented.
Abbreviations: FC, flow cytometry; IWB, immediate whole blood; IPLT, immediate platelets; MFI, mean fluorescence intensity; FITC, fluorescein-5-isocyanate; PE, phycoerythrin; FSC, forward scatter; SSC, side scatter.

|
When analyzing lymphocytes, both fluorescence 1 (FL1) and FL2 photomultipliers were adjusted by running an isotype control prior to a negative control or a sample tube, so that > 90% of the lymphocyte fluorescence signals would be in the first decade counter on the FL1 vs. FL2 plot [
4]. Scatter signals were acquired using linear amplification on a forward scatter (FSC) vs. side scatter (SSC) plot using a lymphocyte gate. A total of ~10,000 events of gated lymphocytes were acquired per tube.
When analyzing platelets, FL1 and FL2 photomultipliers were adjusted for platelets as described above for the lymphocytes. However, scatter signals were acquired using logarithmic amplification on a FSC vs. SSC plot using a platelet gate. A total of ~20,000 events of gated platelets were acquired per tube.
9) Data analysis
Logarithmically amplified fluorescence signals were expressed as linear values (not channel values) (
Fig. 1). Levels of antibody binding to lymphocytes or platelets were measured as mean fluorescence intensities (MFI) within markers set equidistantly around the peak maxima. The upper and lower ends of the markers were set to maximally cover the symmetric portion of the peak.
In this study, MFI "ratio," rather than MFI value, was considered for the HLA-B27 assignment. Three ratios were calculated per sample as follows:
- MFI ratios:
- B7/B27 ratio=B7 MFI ratio/ B27 MFI ratio
3. DNA-based HLA-B27 typing
Real-time PCR was performed for melting curve analysis by using a Real-Q HLA-B*27 detection kit (BioSewoom, Seoul, Korea) [
17] on an ABI 7500 real-time PCR system (Applied Biosystems, Foster city, CA, USA).
4. HLA-A/B/DR typing
Typing was performed using Dynal RELI SSO HLA typing kits (Dynal A.S, Oslo, Norway), which use the PCR-reverse sequence specific oligonucleotide probe (SSOP) method.
Go to :

DISCUSSION
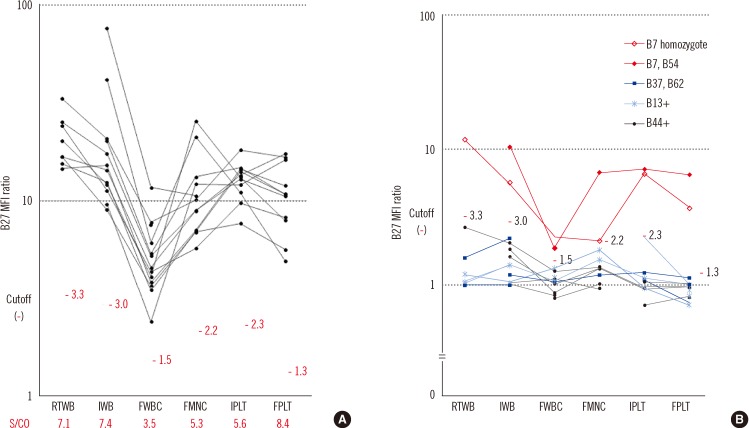
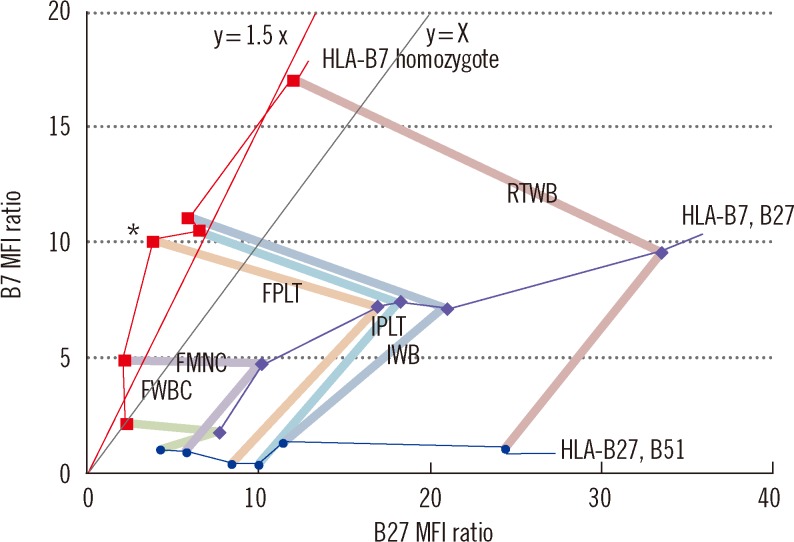
Sample storage is a prerequisite for batch testing. Our study provides an evaluation of various simple storage methods for FC HLA-B27 typing, and shows that FPLT is the best of the four methods examined, with a sensitivity of 100% and a specificity of 99.3% in 164 samples compared with DNA typing as the reference. In "standard" FC HLA-B27 typing, the cells used for analysis are restricted to lymphocytes. However, in the present study, we found that platelets have sufficient HLA molecules for testing.
Out of the 164 samples, FPLT produced a false-positive result in a case with the HLA- B61, B67 phenotype. McKenna and Takemoto [
18] classified HLA-B61 and B67 antigens as the same CREG (B07C CREG); however, Rodey et al. [
11] classified them as different CREGs (B7 and B8 CREG, respectively). IWB also produced a false-positive result for this sample. Particular combinations of non-B27 HLA-B antigens are known to cause false-positive reactions [
19]. In contrast, the HLA-B27+ sample with the lowest B27 MFI ratio (5.3) achieved by FPLT was obtained from a patient with aplastic anemia who had undergone hematopoietic stem cell transplantation; this sample also resulted in a relatively low B27 MFI ratio (14.0) by IWB.
It has been reported that FC HLA-B27 typing can be performed on blood samples stored for up to 16 days at room temperature. In that study, no erroneous HLA-B27 assignment was made for 39 blood samples held for 11 to 16 days [
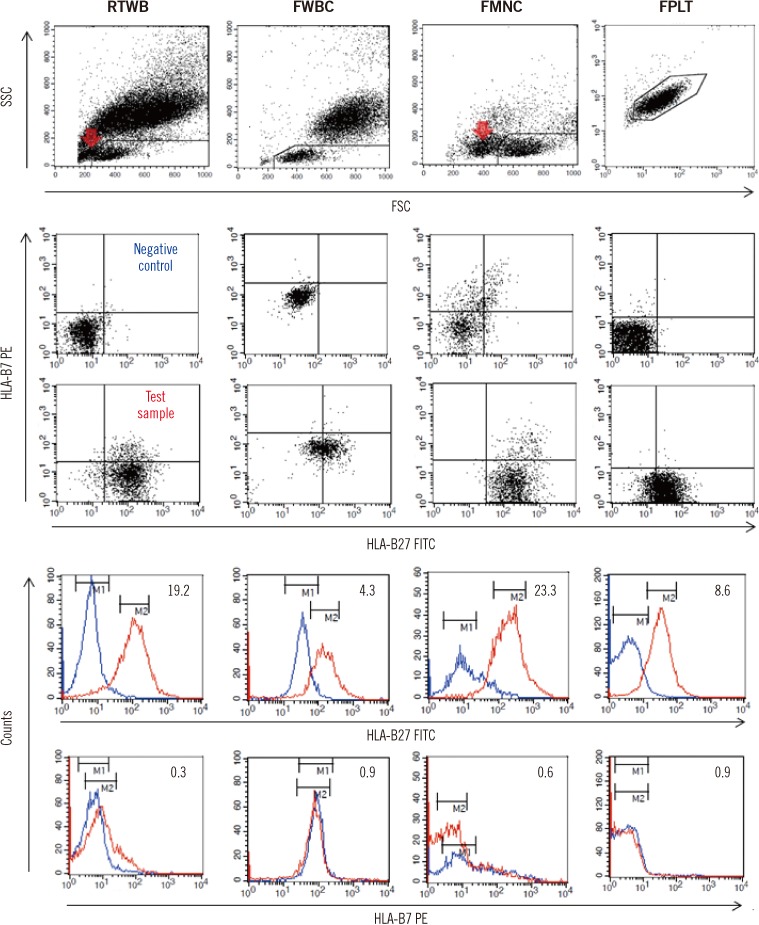
19]. In the present study, blood samples could be held for four days as RTWB also showed no apparent changes in the MFI ratios. However, there is little guarantee of accuracy in samples containing high levels of dead cell debris, as occurred in the case depicted in
Fig. 5, or when contaminating bacteria proliferate during storage. To analyze pure viable lymphocytes in such cases, a T-cell marker such as anti-CD3 should be incorporated into the fluorescent staining protocol [
19].
Although FMNC had a high discriminatory power, it required the most complex procedure, involving MNC isolation, freezing, thawing, and the adjustment of cell counts, to the extent that the method would be too cumbersome for routine clinical laboratory application. FWBC is much simpler than FMNC, but the non-specific binding of anti-B27 and anti-B7 antibodies was pronounced even in negative controls (
Fig. 2,
3A), which markedly diminished its discriminatory power; therefore, this method cannot be recommended.
The processing required for FPLT is much simpler than that required for FMNC: 1) platelets can be simply isolated by differential centrifugation (neither RBC lysing solution nor density-gradient centrifugation is required), 2) platelets can be frozen and thawed in plasma (requiring neither cryosolution nor thawing solution), and 3) FPLT does not require the platelet counts to be adjusted. The present study shows that platelets procured using the FPLT protocol retain patent HLA antigenicity, and that FPLT had the greatest discriminatory power. Therefore, we suggest FPLT as the method of choice for stored samples.
If FC HLA-B27 typing were performed on Friday, a sample referred on Monday would be stored for four days. This is why "four days" was adopted as the storage period in the present study. However, the same conclusions for FPLT would have been reached had the PRP storage period been extended, because freezing and thawing steps more substantially affect cell viability than storage period when a deep freezer is used. It has already been reported that the platelet surface glycoproteins such as HLA and the human platelet antigen (HPA)-1a are stable and are clearly detected by their corresponding antibodies when stored for 12 months at -80℃ [
19].
Although a minimum of two HLA-B27 monoclonal antibody reagents is required to guarantee consistently reliable HLA-B27 typing [
20], we used only a single reagent because our aim was to search for the best storage method, and because only two HLA-B27 alleles (HLA-B*27:04 and B*27:05) are found in the Korean patients with spondyloarthropathies [
21,
22]. Nevertheless, we suppose that the diagnostic efficiency of FPLT would have been improved had other reagents been incorporated.
Although absolute MFI values have been directly compared for the HLA-B27 assignment in the majority of studies [
20], we utilized the MFI "ratio" in the present study. This normalization of test samples versus a negative control allows comparisons of measured values between different cell types (lymphocytes and platelets) and between different batches. However, we suggest that each laboratory defines its own cutoff values, because even MFI ratios are subject to variation between laboratories.
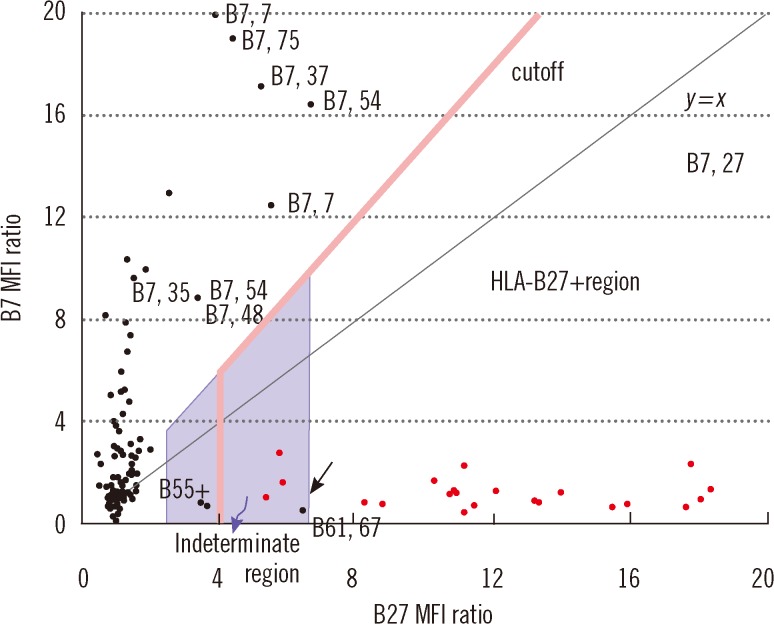
The cutoff B27 MFI ratio should be defined by a statistical analysis of different known samples, including B7 CREG and non-B7 CREG samples. In the present study, the cutoff was defined as 4.0 based on the results of a ROC curve analysis of the 164 samples. Furthermore, when a sample had a B7/B27 ratio of <1.5, a B27 MFI ratio ranging from 2.4 to 6.6 was regarded "indeterminate." This range was defined based on the following statistics: 1) the lower limit, 2.4, was calculated as (the average of 78 HLA-B27- samples with a B27 MFI ratio of <4.0)+(3×SD); and 2) the upper limit, 6.6, was calculated as (the average of 21 HLA-B27+ samples excluding the four outliers)-(2×SD). Therefore, on the B27 vs. B7 MFI ratio plot (
Fig. 6), an "indeterminate" region was defined as that defined by 1) 2.4 <x(B27 MFI ratio) <6.6 and 2) below the line y=1.5x. Samples plotted within this region cannot be accurately determined for HLA-B27 assignment. In the present study, these samples accounted for 3.7% (6/164), and thus, the HLA-B27 assignment would have required complementary DNA typing [
23], which could have been performed using previously frozen blood samples without re-sampling, if the blood cells remaining after removing PRP had been frozen for possible further testing.
It is difficult to discriminate HLA-B7, B27 from other HLA-B7+ samples. When a B7 MFI value of >4 was obtained on a Coulter Epics XL-MCL flow cytometer (Beckman Coulter), Coates and Darke [
9] applied a cutoff B27 MFI (not MFI "ratio") for this discrimination, that is, a B27 MFI of >10 for the former, and a B27 MFI of <5 for the latter. In the present study, we applied a cutoff B7/B27 ratio defined as the midpoint between two groups of restricted sample size. If a statistical approach had been used on a larger number of samples, the cutoff and indeterminate range would have been determined more accurately.
In conclusion, of the four storage methods investigated, none of which required a preserving solution, FC HLA-B27 typing using frozen platelets was found to be the most straightforward, economical, and accurate for both batch testing and control procurement. When stored samples are used, this method has the potential to replace the standard FC typing method. However, some samples (3.7%) with indeterminate results must be confirmed using a complementary DNA-based method.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download