INTRODUCTION
Antifungal susceptibility testing against
Candida is now recognized as a useful aid in patient management and resistance surveillance [
1-
3]. There are 2 independent standards for antifungal susceptibility testing against
Candida: the broth microdilution (BMD) method developed by the Clinical and Laboratory Standards Institute (CLSI) in the United States and the BMD method of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) in Europe [
4,
5]. These 2 methods are similar in that both use the BMD, while they differ in terms of inoculum size and minimum inhibitory concentration (MIC) endpoint determination [
1]. Although both the CLSI and EUCAST have established clinical breakpoints (CBPs) for fluconazole and voriconazole, the EUCAST CBPs are species specific and apply only to
Candida albicans,
Candida tropicalis, and
Candida parapsilosis [
1,
6,
7]. The CLSI is currently revising CBPs for different
Candida species [
1-
3]. The originally proposed CLSI CBPs were not species specific, and assigned values for the susceptibility to ≤8 µg/mL fluconazole and ≤1 µg/mL voriconazole were applied to all
Candida species [
4]. These CBPs are flawed in that a breakpoint is too high to provide a sensitive means of predicting the emergence of resistance among more susceptible species such as
C. albicans,
C. tropicalis, and
C. parapsilosis and simultaneously bisects the wild-type distribution of
Candida glabrata [
1,
3]. Therefore, the CLSI subcommittee reconsidered the MIC distributions for each species and antifungal agent, and developed epidemiological cutoff values (ECVs); these ECV data were used in conjunction with molecular, pharmacodynamic, and clinical data to revise the CBPs to provide species-specific interpretative criteria [
1-
3]. ECVs may also be used to identify isolates that are less likely to respond to antimicrobial therapy because of acquired resistance mechanisms when limited clinical data preclude the development of CBPs [
3].
The EUCAST first defined the wild-type (WT) MICs and ECVs for the 5 most common
Candida species:
C. albicans,
C. tropicalis,
C. parapsilosis,
C. glabrata, and
Candida krusei [
1,
6,
7]. The WT MIC distribution is defined as the MIC distribution for isolates that exhibit no acquired or mutational resistance to the drug in question; meanwhile, non-WT isolates may possess acquired or mutational resistance mechanisms [
1,
6]. The upper limit of the WT population is defined as the ECV. ECVs have recently been applied to EUCAST and CLSI antifungal susceptibility testing, and WT distributions from large collections of the 5 most common
Candida species show that the CLSI and EUCAST methods yield similar ECVs [
1]. While CBPs are used to identify isolates likely to respond to treatment with a given antimicrobial agent administered at its approved dosing regimen, the ECV may serve as the most sensitive measure of the emergence of strains with reduced susceptibility (i.e., acquired resistance) to that agent [
3,
8,
9].
To date, no data on the antifungal susceptibility of Candida bloodstream isolates (BSIs) have been obtained using the EUCAST method or species-specific CBPs or ECVs in Korea. Therefore, we performed a multicenter study to determine the susceptibilities of Candida BSIs to fluconazole and voriconazole in Korea using both the CLSI and EUCAST methods. We also applied the species-specific CBPs and ECVs for Candida BSIs, for the first time. In addition, we compared the CLSI and EUCAST BMD methods for testing 2 azole agents against Candida species using ECVs.
RESULTS
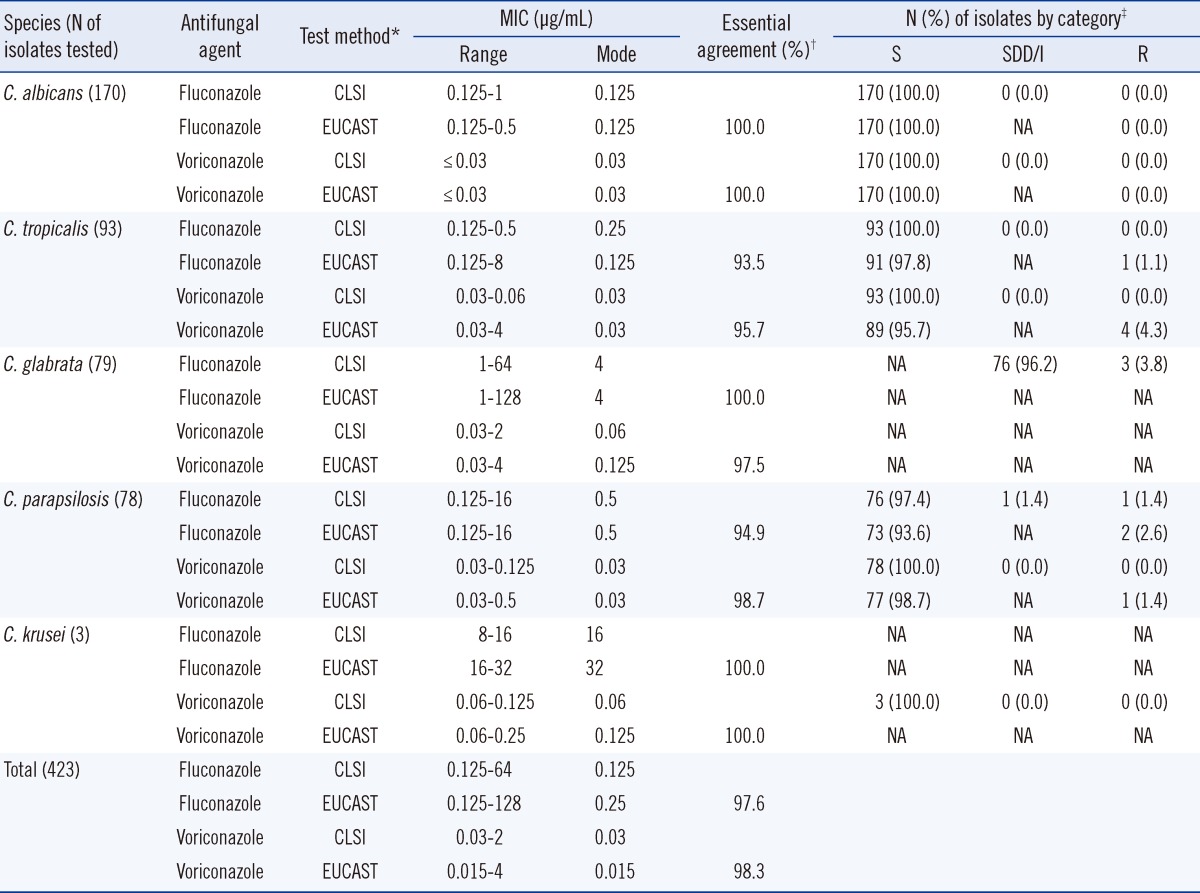
Table 1 summarizes the
in vitro susceptibilities of 423 BSIs of 5
Candida species to fluconazole and voriconazole as determined by the CLSI and EUCAST BMD methods. For all 423 BSIs, the antifungal MIC ranges determined by the CLSI and EUCAST methods were similar: 0.125-64 µg/mL and 0.125-128 µg/mL for fluconazole and 0.03-2 µg/mL and 0.03-4 µg/mL for voriconazole, respectively. For most isolates, the fluconazole and voriconazole MICs obtained by the EUCAST method tended to be one twofold dilution higher than those obtained by the CLSI method. The overall rates of essential agreement (i.e., within 2 dilutions) between the CLSI and EUCAST MIC results were 97.6% for fluconazole and 98.3% for voriconazole. Of the 341 BSIs of the 3 most common species (
C. albicans,
C. tropicalis, and
C. parapsilosis), only 3 (0.9%) and 5 (1.5%) were resistant to fluconazole (MIC ≥8 µg/mL), and voriconazole (MIC ≥0.25 µg/mL) according to the EUCAST method, respectively, and 1 (0.3%) and 0 (0.0%) isolates were resistant to fluconazole (MIC ≥8 µg/mL) and voriconazole (MIC ≥1 µg/mL) according to the CLSI method, respectively. Of the 79
C. glabrata isolates, only 3 (3.8%) were fluconazole resistant (MIC ≥64 µg/mL), while the remaining 76 (96.2%) were SDD (MIC ≤32 µg/mL) according to the CLSI.
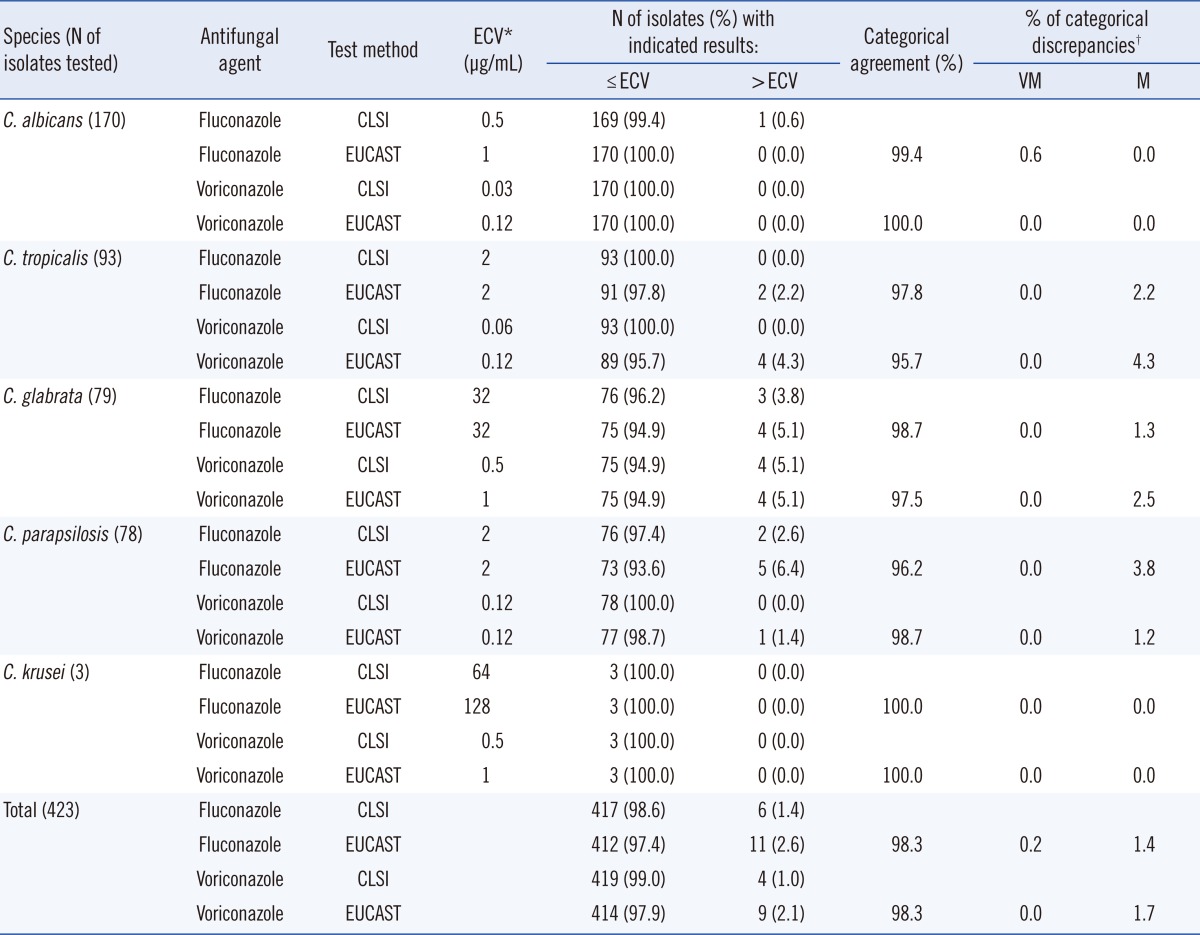
Table 2 shows categorical agreement between the results of the CLSI and EUCAST BMD methods for fluconazole and voriconazole using the ECVs. When the species-specific fluconazole ECVs were applied, the percentages of non-WT isolates determined by the CLSI and EUCAST methods were 0.6% and 0.0% for
C. albicans, 0.0% and 2.2% for
C. tropicalis, 3.8% and 5.1% for
C. glabrata, 2.6% and 6.4% for
C. parapsilosis, and 0.0% and 0.0% for
C. krusei , respectively. In addition, the percentages of non-WT isolates with voriconazole MICs exceeding the ECVs according to the CLSI and EUCAST BMD methods were 0.0% and 0.0% for
C. albicans, 0.0% and 4.3% for
C. tropicalis, 5.1% and 5.1% for
C. glabrata, 0.0% and 1.4% for
C. parapsilosis, and 0.0% and 0.0% for
C. krusei, respectively. Overall, when applying species-specific ECVs, 1.4% and 2.6% of isolates were categorized as fluconazole-resistant non-WT isolates (i.e., exceeding the ECVs) by the CLSI and EUCAST BMD methods, respectively; meanwhile, 1.0% and 2.1% were categorized as voriconazole-resistant non-WT isolates, respectively. The percentages of categorical agreement between the CLSI and EUCAST methods were 98.3% for fluconazole and 98.3% for voriconazole. The overall rates of categorical discrepancies between the EUCAST and CLSI methods were 1.6% for fluconazole (very major errors, 0.2%; major errors, 1.4%) and 1.7% for voriconazole (very major errors, 0.0%; major errors, 1.7%).
DISCUSSION
The CLSI and EUCAST have both published ECVs for the 5 most common
Candida species against fluconazole and voriconazole; they are currently revising their species-specific CBPs for several antifungal agents and various
Candida species to further harmonize both methods [
1-
3]. Although revised species-specific CBPs for both fluconazole and voriconazole are not available for less common
Candida species at present, the CLSI has proposed revised CBPs for 3 common
Candida species by utilizing molecular, microbiologic, pharmacodynamic, and clinical data [
3]. The EUCAST CBPs also apply only to 3 common
Candida species [
6,
7]. When CBPs are unavailable, ECVs can be applied to local and global antifungal surveillance studies to detect the emergence of antifungal resistance among
Candida species [
3,
8,
9]. The present study is the first to compare EUCAST and CLSI MIC results for BSIs of 5 common
Candida species from 8 hospitals in Korea and determine the fluconazole and voriconazole susceptibilities of
Candida BSIs in Korea using species-specific CBPs and ECVs.
In the present study, only 1 (0.3%) isolate of 3 common
Candida species (i.e.,
C. albicans,
C. tropicalis, and
C. parapsilosis) was categorized as fluconazole resistant on the basis of the revised CLSI CBPs; none were categorized as voriconazole resistant. Meanwhile, only 3 (0.9%) and 5 (1.5%) isolates were resistant to fluconazole and voriconazole, respectively, according to the EUCAST method. Several global surveillance programs using CBPs also demonstrate that the majority of BSIs of 3 common
Candida species are susceptible to fluconazole and voriconazole [
10-
13]. However, a recent study indicates a slight but consistent increase in possible resistance to fluconazole and voriconazole among non-WT isolates of 3 common
Candida species according to the EUCAST and CLSI ECVs [
8]. Although
Candida isolates for which the azole MICs exceed the ECV may still respond to clinical treatment as the MIC may lie below the CBP, ECVs can be a sensitive measure for detecting the emergence of
Candida strains with decreased fluconazole and voriconazole susceptibility [
1-
3].
To date, few comparative studies of CLSI and EUCAST antifungal data obtained using ECVs have been performed, except one international study by Pfaller et al. [
14]. This global multicenter study determined the rates of non-WT isolates among 1,056 clinical isolates of 5 common
Candida species to be 3.0% and 5.5% for fluconazole and 2.6% and 4.3% for voriconazole on the basis of ECVs determined by the CLSI and EUCAST methods, respectively. In the present study, the prevalence of non-WT isolates was 1.4% and 2.6% using fluconazole ECVs determined by the CLSI and EUCAST BMD methods, respectively, and 1.0% and 2.1% using voriconazole ECVs, respectively. These results show that the prevalence of non-WT BSIs of
Candida species in Korea remains lower than that in other geographic regions. Pfaller et al. [
15,
16] report that the prevalence of azole resistance among
Candida isolates is variable both over time and by country and region. Although the prevalence of azole resistance among
Candida species is not entirely related to antifungal drug pressure, our previous study provides the first evidence that increased fluconazole use can be significantly correlated with an increased number of fluconazole non-susceptible
Candida isolates from clinical specimens [
17].
C. glabrata is innately less susceptible to azoles than most other
Candida species and rapidly acquires secondary azole resistance following exposure to fluconazole [
18,
19]. The azole resistance frequency among
C. glabrata BSIs may impact the empiric antifungal therapy choice [
15]. The EUCAST has refrained from assigning CBPs for fluconazole and voriconazole to
C. glabrata and advises alternative drugs to manage infections caused by this species [
6,
7]. In contrast to the EUCAST, the CLSI recently revised the CBPs for fluconazole and
C. glabrata to <32 µg/mL for SDD and ≥64 µg/mL for resistant with the caveat that a maximum dose of fluconazole of 800 mg/day (12 mg/kg/day) be used when treating
C. glabrata infections with fluconazole [
3]. When the new CLSI CBPs were applied to the 79
C. glabrata isolates in the present study, 3 (3.8%) and 76 (96.2%) were resistant and SDD to fluconazole, respectively. In a previous global multicenter study, the prevalence of non-WT
C. glabrata isolates according to the ECVs determined by the CLSI and EUCAST methods was 6.9% and 9.7% for fluconazole and 9.1% and 15.4% for voriconazole, respectively [
14]. In the present study, the prevalence of non-WT
C. glabrata isolates according to the ECVs determined by the CLSI and EUCAST methods was 3.8% and 5.1% for fluconazole and 5.1% and 5.1% for voriconazole, respectively. These results show that the azole resistance rates of
C. glabrata BSIs in Korea remain lower than those in other geographic regions.
Similar to the present study, the global multicenter study by Pfaller et al. [
14] demonstrates excellent essential agreement between the CLSI and EUCAST results with respect to susceptibility to fluconazole (98.6% within 2 dilutions) and voriconazole (96.9%). Our data indicate excellent categorical agreement between the CLSI and EUCAST BMD methods for all 5
Candida species for both fluconazole (98.3%) and voriconazole (98.3%). Pfaller et al. [
14] also observed excellent categorical agreement (96%) for all comparisons between the CLSI and EUCAST methods for all
Candida species except
C. parapsilosis (90.8% categorical agreement; 0.6% very major discrepancies). In their study, the prevalence of fluconazole resistance among
C. parapsilosis isolates was relatively high (17.9% and 9.9% determined by the EUCAST and CLSI methods, respectively). In the present study, the categorical agreement between the CLSI and EUCAST methods for
C. parapsilosis was 96.2% and the prevalence of resistant non-WT
C. parapsilosis isolates determined by the CLSI and EUCAST methods was 6.4% and 2.6%, respectively. Therefore, the high categorical agreement in the present study may be due in part to the low frequency of fluconazole resistance in our collection of BSIs of 5
Candida species common in Korea.
In conclusion, our data shows that the EUCAST and CLSI methods using ECVs provide highly concordant results for fluconazole and voriconazole susceptibility in 5 Candida species. Furthermore, the results suggest ECVs are sensitive for detecting the emergence of fluconazole and voriconazole resistance in these Candida species. When we examined the fluconazole and voriconazole susceptibilities of 5 major Candida BSIs using the ECVs, the rates of resistance were found to be low.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download