Abstract
Rothia mucilaginosa is a gram-positive coccus of the family Micrococcaceae. R. mucilaginosa is considered a part of the normal flora of the human oropharynx and upper respiratory tract and lower respiratory tract infections attributable to R. mucilaginosa are not frequent. We present a case of pneumonia, in which the R. mucilaginosa infection was diagnosed by quantitative cultures of a bronchoalveolar lavage (BAL) specimen. A 46-yr-old woman with B lymphoblastic lymphoma was admitted to the hospital for scheduled chemotherapy. Her chest computed tomography (CT) scan revealed bilateral multifocal nodular and patchy consolidation in both lungs. Investigation of the BAL specimen revealed that 7% of leukocytes had intracellular gram-positive cocci. The quantitative cultures of the BAL specimen grew mucoid, non-hemolytic, and grayish convex colonies on blood agar at a count of approximately 200,000 colony-forming units/mL. The colonies were identified as R. mucilaginosa. The patient was empirically treated with levofloxacin for 7 days, after which findings on the chest radiograph and CT scan improved. She was discharged with improvement on hospital day 46. To our knowledge, this is the first report of R. mucilaginosa pneumonia diagnosed in Korea. Quantitative culture of BAL specimen and examination of intracellular organisms are crucial for assessing the clinical significance of R. mucilaginosa recovered from the lower respiratory tract.
Rothia mucilaginosa, previously Stomatococcus mucilaginosus, is an aerobic, gram-positive coccus belonging to the family Micrococcaceae. It is found in the oropharynx and upper respiratory tract as part of the normal flora [1] and was first isolated from an endocarditis patient in 1978 [2]. It is considered an opportunistic pathogen, most often seen in immunocompromised patients, but it is also (less frequently) observed in immunocompetent subjects [3]. There are reports of R. mucilaginosa as a cause of bacteremia, central nervous system infection, meningitis, peritonitis, osteomyelitis, cervical necrotizing fasciitis, endophthalmitis, and endocarditis [1, 2, 4, 5]. Only a dozen cases of lower respiratory tract infection caused by R. mucilaginosa have been diagnosed by recovery of R. mucilaginosa from the bronchoscopic, blood, or sputum specimens [6-9]. R. mucilaginosa is generally considered to be a contaminant in respiratory tract infection specimens. Therefore, for R. mucilaginosa to be confirmed as a cause of a lower respiratory tract infection, the diagnostic specimen must be minimally contaminated. In this study, we report a case of pneumonia due to R. mucilaginosa that was diagnosed by quantitative cultures and visualization of intracellular organisms from a bronchoalveolar lavage (BAL) specimen from a lymphoma patient.
A 46-yr-old woman was diagnosed with B lymphoblastic lymphoma stage IVB on December 6, 2011. She achieved complete remission after 2 courses of chemotherapy that comprised vincristine, prednisolone, daunorubicin, and L-asparaginase. In January 2012, she was treated with ciprofloxacin because of neutropenia and bacteremia caused by Bacillus cereus. On February 18, 2012, fever and diarrhea developed after the second course of chemotherapy, and she received empirical treatment with vancomycin for 4 days and meropenem for 8 days. On February 22, 2012, Clostridium difficile-associated diarrhea developed, and oral metronidazole was administered for 7 days. The patient was admitted to the hospital on March 6, 2012 for scheduled chemotherapy.
On admission, her body temperature was 36.0℃, pulse rate was 101/min, respiration rate was 20/min, and blood pressure was 111/76 mmHg. Complete blood cell count showed a hemoglobin level of 9.6 g/dL, a white blood cell count of 7.5×109/L (with 1.0% myelocytes, 2.0% metamyelocytes, and 54.0% neutrophils), and a platelet count of 351×109/L. The C-reactive protein level was elevated at 0.1 mg/dL. The chest radiograph and computed tomography (CT) scan revealed multifocal nodular and patchy consolidation in both the lungs that suggested atypical pneumonia or bronchiolitis obliterans organizing pneumonia secondary to drug reaction or viral infection (Fig. 1). The abdominal CT scan revealed a liver abscess in liver segment III. Due to suspicion of community-acquired atypical pneumonia, she was treated empirically with levofloxacin for 7 days and underwent bronchoscopy on hospital day (HD) 3.
The BAL specimen was used for the quantitative culture, and a gram-stained smear was prepared with a cytospin centrifuge. Microscopy of the smear revealed a white blood cell count of 10-25/low power field (LPF), an epithelial cell count of <1/LPF, and 7% of leukocytes contained intracellular organisms (ICOs) (Fig. 2). Acid-fast bacilli stain and culture, Mycobacterium tuberculosis PCR, respiratory virus PCR, and fungus culture of the BAL specimen were all negative, but the patient was positive for rhinovirus. The quantitative cultures of the BAL specimen on blood agar plates and chocolate agar plates grew predominantly mucoid, non-hemolytic, and grayish convex colonies after 2 days of incubation at 37℃ and 5% CO2 (Fig. 3A). The colony counts were approximately 200,000 colony-forming units (CFU)/mL. The colonies were sticky on the agar surface. Gram staining of the colonies showed gram-positive cocci of the same morphology as those that appeared on the direct smear (Fig. 3B). The bacterium was identified as R. mucilaginosa using an API Staph Identification Panel (bioMérieux SA, Marcy 1'Etoile, France); the profile number was 6310134 at a probability of 99.9%. The 16S rRNA gene sequencing of this isolate showed an identity of 99.7% (1,325 of 1,329 bp) with R. mucilaginosa ATCC 25296T (GenBank accession no. ACVO01000020). The isolate also had 98.3% homology with R. dentocariosa ATCC 17931T (GenBank accession no. CP002280).
The minimum inhibitory concentrations (MICs) for this isolate were determined using a MicroScan MICroSTREP plus 1 panel (Siemens Healthcare Diagnostics, West Sacramento, CA, USA). This broth microdilution susceptibility test was performed with cation-adjusted Mueller-Hinton agar, supplemented with 3% lysed horse blood (Siemens Healthcare Diagnostics) [10]. MICs of the tested antimicrobials were 0.06 µg/mL for penicillin, 0.06 µg/mL for ampicillin, ≤0.5/0.25 µg/mL for amoxicillin/clavulanate, ≤0.25 µg/mL for azithromycin, >0.5 µg/mL for clindamycin, ≤0.06 µg/mL for erythromycin, 2 µg/mL for chloramphenicol, ≤0.25 µg/mL for ceftriaxone, 1 µg/mL for cefaclor, ≤0.25 µg/mL for cefotaxime, 2 µg/mL for cefepime, ≤0.25 µg/mL for cefuroxime, >4 µg/mL for levofloxacin, >0.5 µg/mL for meropenem, 0.5/9.5 µg/mL for trimethoprim/sulfamethoxazole, 1 µg/mL for tetracycline, and 1 µg/mL for vancomycin. The MICs of other antimicrobials were determined using the E-test (AB Biodisk, Solna, Sweden) on Mueller-Hinton agar supplemented with 5% sheep blood; the MICs were 12 µg/mL for gentamicin, 0.19 µg/mL for imipenem, and 0.5 µg/mL for piperacillin/tazobactam.
Intravenous levofloxacin treatment was stopped on HD 7 because of the possibility of leukopenia induced by antibiotic therapy. The chest radiograph obtained at this time showed a reduction in the multifocal nodular and patchy consolidation in both lungs. Ampicillin/sulbactam and ciprofloxacin were started due to the organizing liver abscess. On HD 30, the third course of chemotherapy was started and completed without complications. The patient was discharged on HD 46 with complete resolution of pneumonia and liver abscess.
In this study, we present a case of pneumonia, in which R. mucilaginosa was the only significant organism recovered from the BAL culture. This intracellular organism was considered to be the causative pathogen of the pneumonia by combined analysis of the quantitative culture and visualization of ICOs. The patient' s chest radiograph and CT scan indicated likely atypical pneumonia, and the BAL specimen was also positive for rhinovirus. Therefore rhinovirus could not be ruled out as a causative agent of pneumonia. However, considering the purulent BAL specimen, we believed that the bacteria played at least a partially pathogenic role, and the patient was treated with antimicrobial therapy.
The predominant growth of R. mucilaginosa in the BAL specimens with rare epithelial cells was not likely due to contamination by the normal oropharyngeal flora. There have been fewer than 20 cases of lower respiratory tract infection caused by R. mucilaginosa reported worldwide [9], and the clinical manifestation of disease have ranged from mild bronchitis to pneumonia or recurrent lung abscesses [6]. However, it is difficult to determine the clinical significance of R. mucilaginosa when it is found in respiratory specimens. The diagnosis of pneumonia caused by R. mucilaginosa requires at least a culture from the bronchoscopic specimen [6, 9]. In this case, the quantitative cultures from the BAL specimen provided more than 105 CFU/mL, which was enough to diagnose the causative agents of pneumonia [11]. These culture findings supported R. mucilaginosa as a true pathogen.
In this case, analysis of the direct smear revealed significant numbers of macrophages and neutrophils engulfing gram-positive cocci in clusters as ICOs. The quantitative cultures of the BAL specimen grew organisms with the same morphology as these ICOs. Detecting ICOs in the BAL fluid is an early indication of an infectious pulmonary process [12, 13], and a cytologic study of the BAL specimen to identify ICOs would allow us to determine the causative agents of nosocomial pneumonia [14]. Quantitative cultures of the BAL specimens show moderate correlation with the percentage of ICOs in the specimen, which in turn correlates with the lung bacterial burden [13]. The percentage of neutrophils that contain ICOs is higher in patients with pneumonia than in those without pneumonia [14]; the cut-off varies from 1% to more than 20% [14]. The Centers for Disease Control and Prevention/National Healthcare Safety Network recommends 5% as a critical value for pneumonia diagnosis [15].
There are no previous reports of pneumonia due to R. mucilaginosa that was diagnosed by quantitative analysis of BAL cultures and ICOs. We identified R. mucilaginosa using the API Staph Identification Panel (bioMérieux SA). Several other commercial kits are available for the identification of R. mucilaginosa; however, their accuracy for this rarely isolated species has been questioned [12, 16]. Because R. mucilaginosa is not a common pathogen in respiratory specimens, we confirmed the identification by 16S rRNA gene sequencing. The morphology of this organism was consistent with its identification as R. mucilaginosa, which is an encapsulated gram-positive coccus found in pairs, clusters, and tetrads, with colonies that are mucoid, rubbery, or sticky in consistency and adherent to agar [1, 16, 17].
The isolate was resistant to gentamicin, clindamycin, and levofloxacin. R. mucilaginosa has a variety of antimicrobial susceptibilities [6, 17-19]. Although the interpretation of antimicrobial susceptibility testing is not standardized, third-generation cephalosporins, vancomycin, high-dose ampicillin, rifampin, and chloramphenicol are consistently active against this bacterium [4, 17]. R. mucilaginosa has a broad range of susceptibility to other agents such as penicillin, clindamycin, and erythromycin [17]. Therefore, the susceptibility pattern of this isolate was generally consistent with that of R. mucilaginosa from previous reports [20, 21]. When the MIC breakpoints for Corynebacterium spp. were applied in this case [10], the organism was susceptible to penicillin, clindamycin, erythromycin, ceftriaxone, cefotaxime, cefepime, meropenem, trimethoprim/sulfamethoxazole, tetracycline, vancomycin, and imipenem. The other antimicrobials were interpreted according to the MIC breakpoints for Staphylococcus spp. [22]; the pathogen was susceptible to ampicillin, amoxicillin/clavulanate, azithromycin, chloramphenicol, cefuroxime, and piperacillin/tazobactam, and resistant to levofloxacin. We can speculate on the emergence of acquired resistance to fluoroquinolones because the patient had a history of ciprofloxacin treatment. There are previous reports of emerging resistance of R. mucilaginosa to ciprofloxacin [18] and trimethoprim/sulfamethoxazole [5] in association with prophylactic antimicrobial therapy.
The variable susceptibility of R. mucilaginosa to β-lactams, aminoglycosides, macrolides, and fluoroquinolones dictates that the choice of antimicrobial agents should be guided by individual susceptibility tests in cases of severe infection. Rifampin, penicillin, ciprofloxacin, gentamicin, and clarithromycin have been previously used to treat R. mucilaginosa pneumonia [6]. Although in our case the isolate was resistant to levofloxacin, the pneumonia improved after levofloxacin therapy. Levofloxacin can be concentrated in respiratory tissues and intracellular compartments [23, 24] and is the treatment of choice for community-acquired pneumonia. In our case, levofloxacin may have been more active than in vitro susceptibility results on ICOs would have led us to expect. Outcomes of R. mucilaginosa pneumonia treated with levofloxacin are generally favorable, but non-attributable mortality has been found in patients with serious underlying disease [6, 7, 9].
In conclusion, this is the first description of pneumonia caused by R. mucilaginosa in Korea. This case indicates that quantitative analysis of BAL culture and ICOs are useful for diagnosing pneumonia caused by R. mucilaginosa.
References
1. Becker K, von Eiff C. Becker K, Christof von EIFF, Bernard KA, Carroll KC, Versalovic J, editors. Staphylococcus, Micrococcus and other catalase-positive cocci. Manual of clinical microbiology. 2011. 10th ed. Washington DC: American Society for Microbiology;p. 308–330.
2. Rubin SJ, Lyons RW, Murcia AJ. Endocarditis associated with cardiac catheterization due to a Gram-positive coccus designated Micrococcus mucilaginosus incertae sedis. J Clin Microbiol. 1978; 7:546–549. PMID: 670378.
3. Faiad G, Singh M, Narasimhan A, Mendez M, Shama S, Nassar N. Rothia mucilaginosa life threatening infections in non-neutropenic hosts. Open J Intern Med. 2011; 1:68–71.
4. Ascher DP, Zbick C, White C, Fischer GW. Infections due to Stomatococcus mucilaginosus: 10 cases and review. Rev Infect Dis. 1991; 13:1048–1052. PMID: 1775836.
5. Treviño M, García-Zabarte A, Quintás A, Varela E, López-Paz JM, Jato A, et al. Stomatococcus mucilaginosus septicemia in a patient with acute lymphoblastic leukaemia. Eur J Clin Microbiol Infect Dis. 1998; 17:505–507. PMID: 9764554.
6. Korsholm TL, Haahr V, Prag J. Eight cases of lower respiratory tract infection caused by Stomatococcus mucilaginosus. Scand J Infect Dis. 2007; 39:913–917. PMID: 17886126.
7. Lambotte O, Debord T, Soler C, Roué R. Pneumonia due to Stomatococcus mucilaginosus in an AIDS patient: case report and literature review. Clin Microbiol Infect. 1999; 5:112–114. PMID: 11856231.
8. Sánchez-Carrillo C, Cercenado E, Cibrián F, Bouza E. Stomatococcus mucilaginosus pneumonia in a liver-transplant patient. Clin Microbiol Newsl. 1995; 16:150–151.
9. Cunniffe JG, Mallia C, Alcock PA. Stomatococcus mucilaginosus lower respiratory tract infection in a patient with AIDS. J Infect. 1994; 29:327–330. PMID: 7884227.
10. Clinical Laboratory Standards Institute. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; Approved guideline 2nd ed, M45-A2. 2010. Wayne, PA: Clinical Laboratory Standards Institute;p. 18–19.
11. Cantral DE, Tape TG, Reed EC, Spurzem JR, Rennard SI, Thompson AB. Quantitative culture of bronchoalveolar lavage fluid for the diagnosis of bacterial pneumonia. Am J Med. 1993; 95:601–607. PMID: 8259777.

12. Allaouchiche B, Jaumain H, Dumontet C, Motin J. Early diagnosis of ventilator-associated pneumonia. Is it possible to define a cutoff value of infected cells in BAL fluid? Chest. 1996; 110:1558–1565. PMID: 8989077.
13. Torres A, El-Ebiary M, Fábregas N, Gonzáilez J, de la Bellacasa JP, Hernández C, et al. Value of intracellular bacteria detection in the diagnosis of ventilator associated pneumonia. Thorax. 1996; 51:378–384. PMID: 8733489.

14. Solé-Violán J, Rodríguez de Castro F, Rey A, Martín-González JC, Cabrera-Navarro P. Usefulness of microscopic examination of intracellular organisms in lavage fluid in ventilator-associated pneumonia. Chest. 1994; 106:889–894. PMID: 8082373.

15. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008; 36:309–332. PMID: 18538699.

16. Garcia LS, Isenberg HD, editors. Clinical microbiology procedures handbook. 2010. 1:3rd ed. Washington DC: ASM Press;3.11.2.173.11.2.20-7.
17. von Eiff C, Herrmann M, Peters G. Antimicrobial susceptibilities of Stomatococcus mucilaginosus and of Micrococcus spp. Antimicrob Agents Chemother. 1995; 39:268–270. PMID: 7695321.
18. von Eiff C, Peters G. In vitro activity of ciprofloxacin, ofloxacin, and levofloxacin against Micrococcus species and Stomatococcus mucilaginosus isolated from healthy subjects and neutropenic patients. Eur J Clin Microbiol Infect Dis. 1998; 17:890–892. PMID: 10052561.
19. Skogen PG, Kolmannskog S, Bergh K. Bactericidal activity in cerebrospinal fluid by treating meningitis caused by Stomatococcus mucilaginosus with rifampicin, cefotaxime and vancomycin in a neutropenic child. Clin Microbiol Infect. 2001; 7:39–42. PMID: 11284946.
20. McWhinney PH, Kibbler CC, Gillespie SH, Patel S, Morrison D, Hoffbrand AV, et al. Stomatococcus mucilaginosus: an emerging pathogen in neutropenic patients. Clin Infect Dis. 1992; 14:641–646. PMID: 1562654.
21. Chomarat M, Vital MG, Flandrois JP. Susceptibility to aminoglycosides of 63 strains of Stomatococcus mucilaginosus isolated from sputum. Zentralbl Bakteriol. 1991; 276:63–67. PMID: 1789902.
22. Clinical Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Twenty-Second Informational supplement,M100-S22. 2012. Wayne, PA: Clinical Laboratory Standards Institute;p. 70–79.
23. Capitano B, Mattoes HM, Shore E, O'Brien A, Braman S, Sutherland C, et al. Steady-state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adults. Chest. 2004; 125:965–973. PMID: 15006955.

24. Zhanel GG, Ennis K, Vercaigne L, Walkty A, Gin AS, Embil J, et al. A critical review of the fluoroquinolones: focus on respiratory infections. Drugs. 2002; 62:13–59. PMID: 11790155.
Fig. 1
(A) Chest radiograph showing bilateral ground glass opacity and patchy nodular opacity in both lungs. (B) Axial contrast-enhanced chest computed tomography image showing multifocal nodular and patchy consolidation in both lungs.
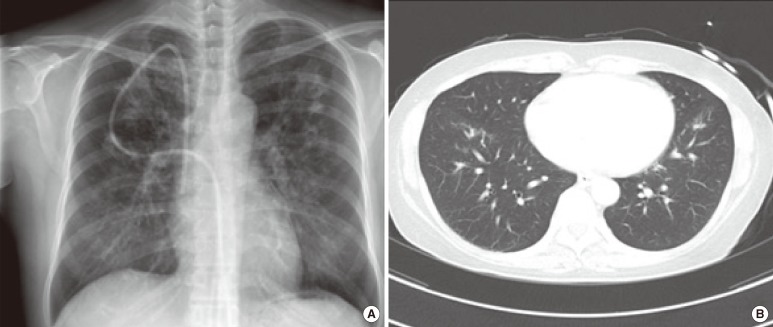
Fig. 2
Microscopic images of gram-stained smears of the bronchoalveolar lavage specimens that were prepared with a cytospin centrifuge (Gram stain, ×1,000). Images show clusters of neutrophils containing intracellular gram-positive cocci. Arrowheads indicate the neutrophil-containing intracellular gram-positive cocci.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download