An anti-hepatitis A virus (HAV) IgM test is crucial to diagnose current HAV infection. Commercialized anti-HAV IgM chemiluminescence immunoassay has been widely used recently because of its significantly improved specificity and technical simplicity [
1], although reports on performance are scarce [
2,
3]. Performance of 3 anti-HAV IgM assays-Architect HAV Antibody (HAVAb)-IgM (Abbott Laboratories, Abbott Park, IL, USA), Elecsys Anti-HAV IgM (Roche Diagnostics, Mannheim, Germany), and ADVIA Centaur HAV IgM (Siemens Healthcare Diagnostics Inc., Deerfield, IL, USA)-was compared under routine conditions in the clinical laboratory of Seoul National University Hospital.
The study included 178 consecutive samples for immediate anti-HAV IgM testing using Architect HAVAb-IgM between September 2009 and January 2010. We collected the remaining sera as aliquots in 1.5 mL tubes immediately after the Architect HAVAB-IgM test and stored them at -80℃ until analysis. Elecsys and ADVIA Centaur HAV IgM were performed on the same day according to the manufacturers' instructions. For Architect, signal-to-cutoff (S/CO) values of 0.80-1.20 were considered gray-zone values. For ADVIA Centaur, an S/CO ≥0.80 and <1.20 was considered equivocal.
Medical records were reviewed, or reverse transcription (RT)-PCR for HAV and ADVIA Centaur total HAV were performed for 16 sera showing discrepant results. RNA was extracted using a Chemagic Viral DNA/RNA preparation kit (Chemagen, Baesweiler, Germany), and RT-PCR was performed using the AccuPower HAV Real-Time RT-PCR kit (Bioneer Corp., Daejeon, Korea). This study was approved by the Seoul National University Hospital Institutional Review Board (E-1110-046-381).
The agreements (kappas) between assays were calculated [
4]. Correlations in S/CO values between assays were evaluated by a Spearman's test, excluding those results exceeding the measurable range using SPSS for Windows (version 12.0; SPSS Inc., Chicago, IL, USA).
Among 178 samples, 45 (25.3%) were reactive and 117 (65.7%) were nonreactive for all 3 kits. When the gray-zone results of Architect and ADVIA Centaur were interpreted as nonreactive, the percent agreements (kappas) between Architect and ADVIA Centaur, Architect and Elecsys, and ADVIA Centaur and Elecsys were 96.6% (0.91), 96.6% (0.92), and 97.8% (0.94), respectively. Among the 16 (9.0%) discrepant sera, 8 (case 1-8,
Table 1) showed gray-zone values with Architect, but they were nonreactive with ADVIA Centaur and Elecsys. The negative anti-HAV IgM follow-up tests indicated that cases 1 and 2 were less likely to have HAV infection. For cases 3-8, HAV infection could not be ruled out from additional test results (HAV RT-PCR, negative; total anti-HAV, reactive). Case 9 (Architect, reactive; others, nonreactive) and Case 10 (ADVIA Centaur, reactive; others, nonreactive) were also less likely to have HAV infection considering the negative HAV RT-PCR, although very high levels of AST and ALT were seen.
Table 1
Clinical characteristics of cases with discrepant results among Architect, ADVIA Centaur, and Elecsys Anti-HAV IgM assays (N=16)
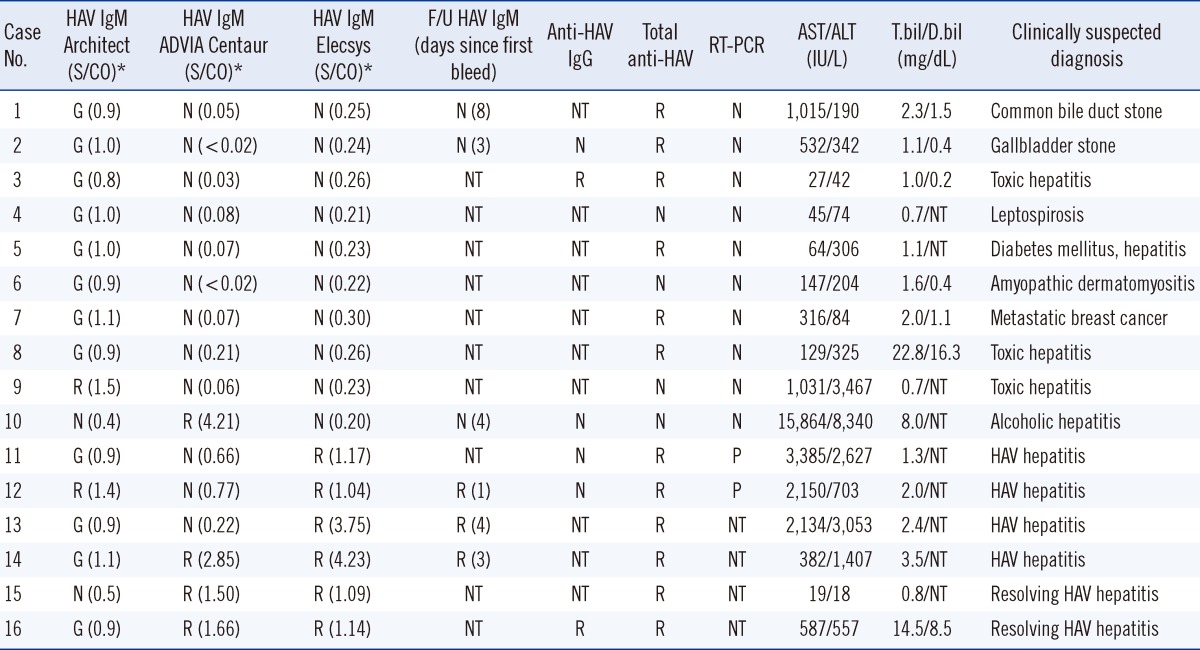

Cases 11 and 12, confirmed as HAV+ (positive RT-PCR), were nonreactive with ADVIA Centaur but reactive with Elecsys. Cases 13 and 14, confirmed as HAV+ from reactive results with higher S/CO values of follow-up anti-HAV IgM tests in all 3 assays, showed gray-zone results with Architect and were reactive with Elecsys. Case 13 was nonreactive with ADVIA Centaur.
Cases 15 and 16, with infection history (7 and 8 months ago, respectively), (reactive anti-HAV IgM and clinical course consistent with HAV infection) were reactive with ADVIA Centaur and Elecsys and nonreactive and in the gray-zone with Architect, respectively.
Although, these assays were not quantitative, their S/CO values were moderately correlated with each other. Spearman's correlation coefficient (r) between Architect and the ADVIA Centaur HAV IgM was 0.757 (
P<0.001); Architect and Elecsys, 0.732 (
P<0.001); and Elecsys and ADVIA Centaur, 0.776 (
P<0.001) (
Fig. 1).
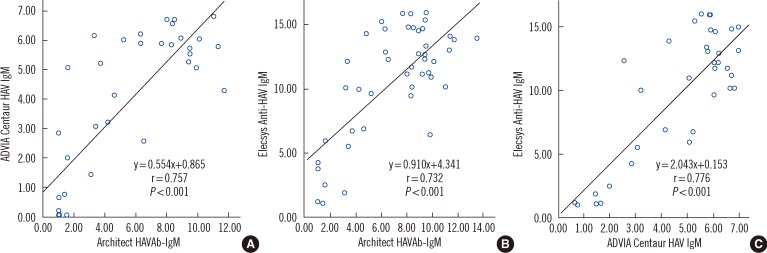 | Fig. 1
Correlations of signal-to-cutoff (S/CO) values among Architect HAVAb-IgM, ADVIA Centaur HAV IgM, and Elecsys Anti-HAV IgM assays. (A) Scatter plot of S/CO values of Architect HAVAb-IgM and ADVIA Centaur HAV IgM assays, (B) Architect HAVAb-IgM and Elecsys Anti-HAV IgM assays, and (C) ADVIA Centaur HAV IgM and Elecsys Anti-HAV IgM assays.
Abbreviation: HAV, hepatitis A virus.

|
Here, 3 kits showed excellent overall agreement (kappas: 0.91-0.94) when the gray-zone values of Architect were considered nonreactive (ADVIA Centaur showed no equivocal results). Architect showed gray-zone results in 12 samples: HAV infections, 4; less-likely infections, 2; uncertain for infection, 6. The agreement was slightly lower (kappa values: Architect and ADVIA Centaur, 0.81; Architect and Elecsys, 0.87; data not shown) when the gray-zone values of Architect were considered reactive.
ELISAs can exhibit false-reactive results in various conditions, including autoimmune diseases or renal failure [
5]. Rheumatoid factor or heterophilic antibodies can also interfere with immunoassay results [
6,
7]. Nonspecific binding of serum IgM to the microparticle bead induces false reactivity in the Liaison system adopting chemiluminescence immunoassay, in the absence of rheumatoid factor or paraprotein; the use of chemical-blocking reagents eliminated this problem [
8]. The Architect system adopts a different assay principle (direct coating of HAV antigens on a microparticle bead) from that of the other assays (using streptavidin-coated microparticles and biotinylated mouse anti-human IgM antibodies). Further investigations are needed to determine if gray-zone results, more frequently observed with Architect, could be partially explained by the nonspecific adsorption of proteins to the microparticle bead.
In cases 11-14, in the early phase of HAV infection, the ADVIA Centaur and Architect showed slightly later seroconversions compared to the Elecsys. Two cases with history of HAV infection (~7-8 months ago) were reactive with ADVIA Centaur and Elecsys with low S/CO values (1.09-1.66), whereas Architect showed nonreactive in one sample and gray-zone in another, suggesting a slight difference in the sensitivity for the detection of decreasing anti-HAV IgM in patients who had recovered from previous HAV infection.
Although all 3 kits are not quantitative tests, the S/CO values showed moderate correlations among them. For samples from patients with resolving HAV infection, S/CO values were low (1.09-1.66), suggesting very low anti-HAV IgM levels. Further development of quantitative tests for anti-HAV IgM may be helpful in patients showing atypical disease courses during HAV infection or HAV reactivation after transplantation [
9].
In conclusion, 3 automated immunoassay kits showed comparable performances, with excellent overall agreement among them when performed on samples submitted to a tertiary care hospital and can be successfully applied in clinical laboratory practice.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download