Abstract
Background
Shigella is a frequent cause of bacterial dysentery in the developing world. Treatment with antibiotics is recommended for shigellosis, but the options are limited due to globally emerging resistance. This study was conducted to determine the frequency and pattern of antimicrobial susceptibility of Shigella in China.
Methods
We studied the antimicrobial resistance profiles of 308 Shigella spp. strains (260 S. flexneri, 40 S. sonnei, 5 S. boydii, and 3 S. dysenteriae) isolated from fecal samples of patients (age, from 3 months to 92 yr) presenting with diarrhea in different districts of Anhui, China. The antimicrobial resistance of strains was determined by the agar dilution method according to the CSLI guidelines.
Results
The most common serogroup in the Shigella isolates was S. flexneri (n=260, 84.4%), followed by S. sonnei (n=40, 13.0%). The highest resistance rate was found for nalidixic acid (96.4%), followed by ampicillin (93.2%), tetracycline (90.9%), and trimethoprim/sulfamethoxazole (80.8%). Among the isolates tested, 280 (91.0%) were multidrug resistant (resistant to ≥2 agents). The most common resistance pattern was the combination of ampicillin, tetracycline, and trimethoprim/sulfamethoxazole (70.8%). Resistance to ampicillin and tetracycline were more common among S. flexneri than among S. sonnei isolates.
Conclusions
S. flexneri is predominant in Anhui, China, and its higher antimicrobial resistance rate compared with that of S. sonnei is a cause for concern. Continuous monitoring of resistance patterns is necessary to control the spread of resistance in Shigella. The recommendations for antimicrobial treatment must be updated regularly based on surveillance results.
Shigellosis is an acute enteric infection caused by Shigella species and is manifested by diarrhea. Shigellosis is endemic to many developing countries, and it causes considerable morbidity and mortality [1, 2]. It was reported that more than 140 million cases of shigellosis occurred worldwide, with 600,000 people dying annually; 60% of the deaths were observed in children aged less than 5 yr [3]. In China, 0.8-1.7 million episodes of shigellosis were reported in 2000, and the predominant species was Shigella flexneri [4]. S. flexneri is the most commonly isolated Shigella species in the developing world and the most frequent cause of bacterial dysentery. Despite the disease being self-limiting, antimicrobial agents are recommended for the treatment of shigellosis, because it reduces the duration of the illness and the transmission rate of the disease by shortening the period required for excretion of the pathogen [5]. However, Shigella species have developed antimicrobial resistance since 1940, when the resistance of Shigella species to sulfonamide was first recognized in Japan [6].
In recent decades, Shigella species have become progressively more resistant to most of the widely-used antimicrobial agents, resulting in reduced efficacy of antimicrobial therapies [6, 7]. The increasing levels of antimicrobial resistance in Shigella isolates have complicated the selection of empirical agents for its treatment. Moreover, antimicrobial resistance among Shigella spp. varies from region to region. Thus, when choosing an appropriate antibiotic to treat shigellosis, it is important to understand the local antimicrobial resistance. We conducted the present study to determine the prevalence of Shigella species and their antimicrobial resistance patterns in China in order to better manage shigellosis.
From 2005 to 2011, a total of 308 non-duplicate Shigella isolates (260 S. flexneri, 40 S. sonnei, 5 S. boydii, and 3 S. dysenteriae) were isolated from the stool samples of different patients at 34 hospitals in Anhui Province, China. The patients included in this study were from 18 different districts in Anhui Province, including Hefei, Wuhu, Bengbu, Huainan, Ma'anshan, Huaibei, Tongling, Anqing, Huangshan, Chuzhou, Fuyang, Suzhou, Lu'an, Bozhou, Chizhou, Chaohu, and Xuancheng, which are distributed across the province. Individual isolates were identified by standard microbiological and biochemical methods and were confirmed using the API-20E system (bioMérieux, Marcy l' Étoile, France). All isolates were serotyped using commercial antisera (Denka Seiken Co. Ltd., Tokyo, Japan).
Minimal inhibitory concentrations of the following 19 antimicrobial agents were determined by the agar dilution method according to the CLSI guidelines [8]: ampicillin (AMP), piperacillin (PIP), cefotaxime (CTX), ceftriaxone (CRO), ceftazidime (CAZ), cefepime (FEP), cefoxitin (FOX), aztreonam (ATM), nalidixic acid (NAL), ciprofloxacin (CIP), levofloxacin (LEV), norfloxacin (NOR), gatifloxacin (GAT), gentamicin (GM), amikacin (AMK), chloramphenicol (CHL), trimethoprim/sulfamethoxazole (SXT), tetracycline (TET), and imipenem (IMP). Escherichia coli ATCC 25922 was used for quality control. Susceptible and non-susceptible isolates were defined according to the criteria used for Enterobacteriaceae suggested by the CLSI [8].
Statistical analysis focused on the relationships between antimicrobial resistance patterns and Shigella serotype, geographic location, and age and sex of the patient. We defined multidrug resistance as resistance to 2 or more classes of antimicrobial agents among all classes included in the analysis [9]. In order to calculate incidence rates by geographic region, we grouped the 17 participating districts into 3 regions: South (Wuhu, Ma'anshan, Tongling, Huangshan, Chizhou, and Xuancheng), North (Bengbu, Huaibei, Fuyang, Suzhou, and Bozhou), and Mid (Hefei, Huainan, Anqing, Chuzhou, Lu'an, and Chaohu).
The SPSS (Statistical Product and Service Solutions, version 13.0) (SPSS Inc., Chicago, IL, USA) was used for the analysis of data. We used the χ2 test to compare proportions and Fisher's exact test when appropriate. P values were based on two-tailed test results, and P values of <0.05 were considered statistically significant.
Among the 308 isolates, S. flexneri was the most prevalent species (n=260, 84.4%), followed by S. sonnei (n=40, 13.0%), S. boydii (n=5, 1.6%), and S. dysenteriae (n=3, 1.0%). S. flexneri accounted for 60.0% of all Shigella isolates from the South region, 93.2% from the North region, and 60.5% from the Mid region. S. sonnei accounted for 40.0% of all Shigella isolates from the South region, 4.3% from the North region, and 37.2% from the Mid region. Among the 5 S. boydii isolates, 4 were from the North region, and 1 was from the Mid region. All 3 S. dysenteriae isolates were from the North region.
Shigella strains were isolated from specimens obtained from symptomatic patients in all age groups. The median age was 4 yr (range, from 3 months to 92 yr), and 53.0% of the subjects were male. The patients from whom S. sonnei was isolated were younger than those from whom S. flexneri was isolated (median age, 4 yr vs. 8 yr; P<0.05). Infants and children 1-4 yr accounted for the highest proportion of S. flexneri (29%) and S. sonnei (59.7%) isolates. Overall, more number of S. flexneri isolates were obtained from male subjects than from female subjects (53.5% vs. 46.5%; P<0.05).
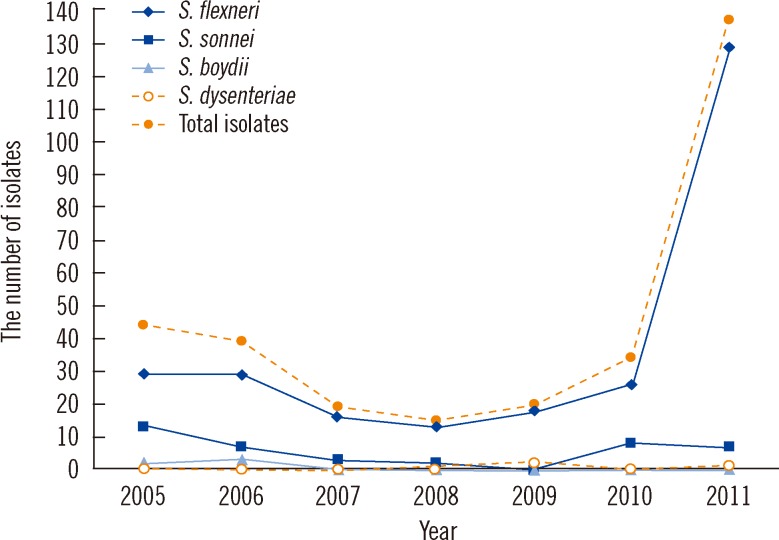
Fig. 1 shows the yearly distribution of the Shigella isolates. S. flexneri was the most common species isolated each year. A sharp increase in the number of S. flexneri isolates was observed in 2011, i.e., from 26 in 2010 to 129. S. boydii was only isolated in 2005 (n=2) and 2006 (n=3), and S. dysenteriae was only isolated once per year in 2005, 2009, and 2011.
A comparative analysis of the sensitivities of the Shigella isolates against the individual antibiotics was performed. The highest resistance rate was found for NAL (96.4%), followed by AMP (93.2%), TET (90.9%), and SXT (80.8%). Overall, 94.2% of the S. flexneri strains and 85.0% of S. sonnei strains were resistant to AMP; 96.2% of the S. flexneri strains and 65.0% of S. sonnei strains were resistant to TET, and 81.9% of the S. flexneri strains and 75.0% of the S. sonnei strains were resistant to SXT. S. flexneri was most sensitive to IMP (100%), followed by FOX (99.2%) and CAZ (97.7%). S. sonnei was most sensitive to IMP (100%), followed by AMK (95.0%), GAT (90.0%), ATM (90.05.5%), and FEP (90.0%).
Among the 308 isolates tested, 280 (91.0%) were multidrug resistant (resistant to 2 or more agents). The most common resistance pattern was the combination of AMP, TET, and SXT (70.8%). Resistance to the combination of AMP, CIP, and SXT was observed in 74 isolates (24.0%). Resistance to the combinations of AMP, TET, and SXT, or to that of AMP, CIP, and SXT were similar between the S. flexneri and S. sonnei isolates (73.1% and 60.0%, P=0.09; 23.1% and 27.5%, P=0.54, respectively).
Resistance to AMP was more common among S. flexneri isolates than that among S. sonnei isolates (94.2% vs. 85.0%, P=0.03); resistance to TET was also more common among S. flexneri isolates than that among S. sonnei isolates (96.2% vs. 65.0%, P=0.00). However, the proportions of S. flexneri and S. sonnei isolates resistant to SXT were similarly high (81.9% and 75.0%, P=0.30) (Table 1).
Global studies have suggested that 164.7 million people suffer from shigellosis every year. The number of deaths due to shigellosis has been estimated to be 1.1 million annually, particularly among children in developing countries [1]. S. sonnei is the predominant cause of shigellosis in developed countries, and it is more common in children than in adults [10, 11]. However, in developing countries with low socioeconomic conditions, S. flexneri is the predominant species. In China, 0.8-1.7 million episodes of shigellosis were reported in 2000, and the predominant species was S. flexneri [4], which was consistent with the findings of our study.
Increasing antimicrobial resistance has complicated the selection of empirical antibiotics for the treatment of shigellosis. From 1999 to 2002, data from the National Antimicrobial Resistance Monitoring System (NARMS) in the United States revealed a high rate of resistance to AMP (78.0%) and SXT (46.0%), as well as multidrug resistance (64.0%) among Shigella isolates [9]. The multidrug-resistance rate (91.0%) among Shigella isolates in our study was significantly high. Although only limited numbers of strains were examined, these results showed that the level of resistance demonstrated by Shigella in China is serious and that this phenomenon may be due to increasing antibiotic overuse in both humans and animals.
According to the WHO guidelines for the control of shigellosis, CIP is now the drug of choice for all patients with bloody diarrhea, irrespective of their age [2]. However, there have been several previous reports on the evolution of CIP resistance among Shigella strains. CIP resistance was documented in approximately 0.5% of Shigella isolates in Israel from 1998 to 2000 [12], in 0.06% of isolates in the United States from 1999 to 2002 [9], and in 0.2% of isolates in Asia from 2000 to 2004 [13]. In the Asia-Africa area, the rate of resistance to CIP was 29.1% in 2007-2009, while the levels of resistance in isolates from Europe-America remained low (< 1.0%) [14]. In a previous study conducted in 2006 in Henan Province, China, all of the 71 S. flexneri isolates exhibited resistance to NAL; in addition, 21% and 79% showed high- or low-level resistance to CIP, respectively [15]. In this study, a significantly higher rate of resistance to CIP (27.9%: 25.4% for S. flexneri and 35.0% for S. sonnei) was demonstrated when compared with the rate of resistance to LEV (9.7%) (P<0.05). Pu et al. [16] reported that 15 out of 123 S. flexneri isolates showed different levels of resistance to NAL, CIP, and LEV. The rate of resistance to CIP and LEV were 100% and 40.0%, respectively. Our results were similar to their report. This difference may be due to the increasing quinolone overuse in both humans and livestock in China.
Because the proportion of isolates resistant to AMP, CHL, SXT, and TET has increased substantially among Shigella isolates worldwide in the last decade, these drugs are no longer recommended as empirical therapy for shigellosis by the WHO [2]. The high prevalence of antimicrobial resistance among Shigella isolates noted in this study limits the safe and efficacious treatment options for shigellosis, particularly for children [17-19]. Because resistance to AMP, TET, and SXT is common, appropriate antimicrobial agents for the treatment of shigellosis are limited to fluoroquinolones, CAZ, CRO, and AMK according to our study.
In conclusion, our study demonstrates that S. flexneri is currently the predominant species in Anhui, China, and its higher antimicrobial resistance rate compared with that of S. sonnei is a cause for concern. The antimicrobial resistance pattern suggests widespread resistance of Shigella to AMP, TET, and SXT. Empirical antimicrobial therapy for shigellosis requires knowledge of the antimicrobial resistance pattern of local Shigella strains. Clinical practitioners should be aware of the high multidrug resistance of Shigella spp., particularly the increasing resistance to fluoroquinolones. Continuous monitoring of resistance patterns at the national and international levels is required in order to control the spread of resistance in Shigella. The recommendations for antimicrobial treatment must be updated regularly based on surveillance results.
Acknowledgements
We are indebted to the 34 participating hospitals for their assistance in collecting the isolates. The authors thank Huimin Ma, Xue Zhou, Liguang Liu, and Xiuying Miao for their technical assistance in antimicrobial susceptibility testing.
This study was supported by the Natural Science Foundation of China (No. 30972631) and the Provincial Natural Science Foundation Key Program of Higher Education of China (No. KJ2010A344).
References
1. Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999; 77:651–666. PMID: 10516787.
2. Legros D, editor. Guidelines for the control of shigellosis, including epidemics due to Shigella dysenteriae type 1. 2005. Geneva: World Health Organization;http://www.who.int/vaccine_research/documents/Guidelines_Shigellosis.pdf.
3. Sur D, Ramamurthy T, Deen J, Bhattacharya SK. Shigellosis: challenges & management issues. Indian J Med Res. 2004; 120:454–462. PMID: 15591629.
4. Wang XY, Tao F, Xiao D, Lee H, Deen J, Gong J, et al. Trend and disease burden of bacillary dysentery in China (1991-2000). Bull World Health Organ. 2006; 84:561–568. PMID: 16878230.

5. Bhattacharya SK, Sur D. An evaluation of current shigellosis treatment. Expert Opin Pharmacother. 2003; 4:1315–1320. PMID: 12877639.

6. Watanabe T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963; 27:87–115. PMID: 13999115.

7. Sack RB, Rahman M, Yunus M, Khan EH. Antimicrobial resistance in organisms causing diarrheal disease. Clin Infect Dis. 1997; 24:S102–S105. PMID: 8994788.

8. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Twenty-second Informational supplement, M100-S22. 2012. Wayne, PA: Clinical and Laboraotory Standards Institute.
9. Sivapalasingam S, Nelson JM, Joyce K, Hoekstra M, Angulo FJ, Mintz ED. High prevalence of antimicrobial resistance among Shigella isolates in the United States tested by the National Antimicrobial Resistance Monitoring System from 1999 to 2002. Antimicrob Agents Chemother. 2006; 50:49–54. PMID: 16377666.
10. Ashkenazi S, May-Zahav M, Dinari G, Gabbay U, Zilberberg R, Samra Z. Recent trends in the epidemiology of Shigella species in Israel. Clin Infect Dis. 1993; 17:897–899. PMID: 8286636.

11. DeLappe N, O'Halloran F, Fanning S, Corbett-Feeney G, Cheasty T, Cormican M. Antimicrobial resistance and genetic diversity of Shigella sonnei isolates from western Ireland, an area of low incidence of infection. J Clin Microbiol. 2003; 41:1919–1924. PMID: 12734227.
12. Ashkenazi S, Levy I, Kazaronovski V, Samra Z. Growing antimicrobial resistance of Shigella isolates. J Antimicrob Chemother. 2003; 51:427–429. PMID: 12562716.

13. von Seidlein L, Kim DR, Ali M, Lee H, Wang X, Thiem VD, et al. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med. 2006; 3:e353. PMID: 16968124.

14. Gu B, Cao Y, Pan S, Zhuang L, Yu R, Peng Z, et al. Comparison of the prevalence and changing resistance to nalidixic acid and ciprofloxacin of Shigella between Europe-America and Asia-Africa from 1998 to 2009. Int J Antimicrob Agents. 2012; 40:9–17. PMID: 22483324.

15. Xia S, Xu B, Huang L, Zhao JY, Ran L, Zhang J, et al. Prevalence and characterization of human Shigella infections in Henan Province, China, in 2006. J Clin Microbiol. 2011; 49:232–242. PMID: 21068291.

16. Pu XY, Pan JC, Wang HQ, Zhang W, Huang ZC, Gu YM. Characterization of fluoroquinolone-resistant Shigella flexneri in Hangzhou area of China. J Antimicrob Chemother. 2009; 63:917–920. PMID: 19297378.

17. Xiong Z, Li J, Li T, Shen J, Hu F, Wang M. Prevalence of plasmid-mediated quinolone-resistance determinants in Shigella flexneri isolates from Anhui Province, China. J Antibiot (Tokyo). 2010; 63:187–189. PMID: 20203702.

18. Keddy KH, Sooka A, Crowther-Gibson P, Quan V, Meiring S, Cohen C, et al. Systemic shigellosis in South Africa. Clin Infect Dis. 2012; 54:1448–1454. PMID: 22474223.

19. Taneja N, Mewara A, Kumar A, Verma G, Sharma M. Cephalosporin-resistant Shigella flexneri over 9 years (2001-09) in India. J Antimicrob Chemother. 2012; 67:1347–1353. PMID: 22410619.

Table 1
Antimicrobial resistance of Shigella isolates in China from 2005 to 2011
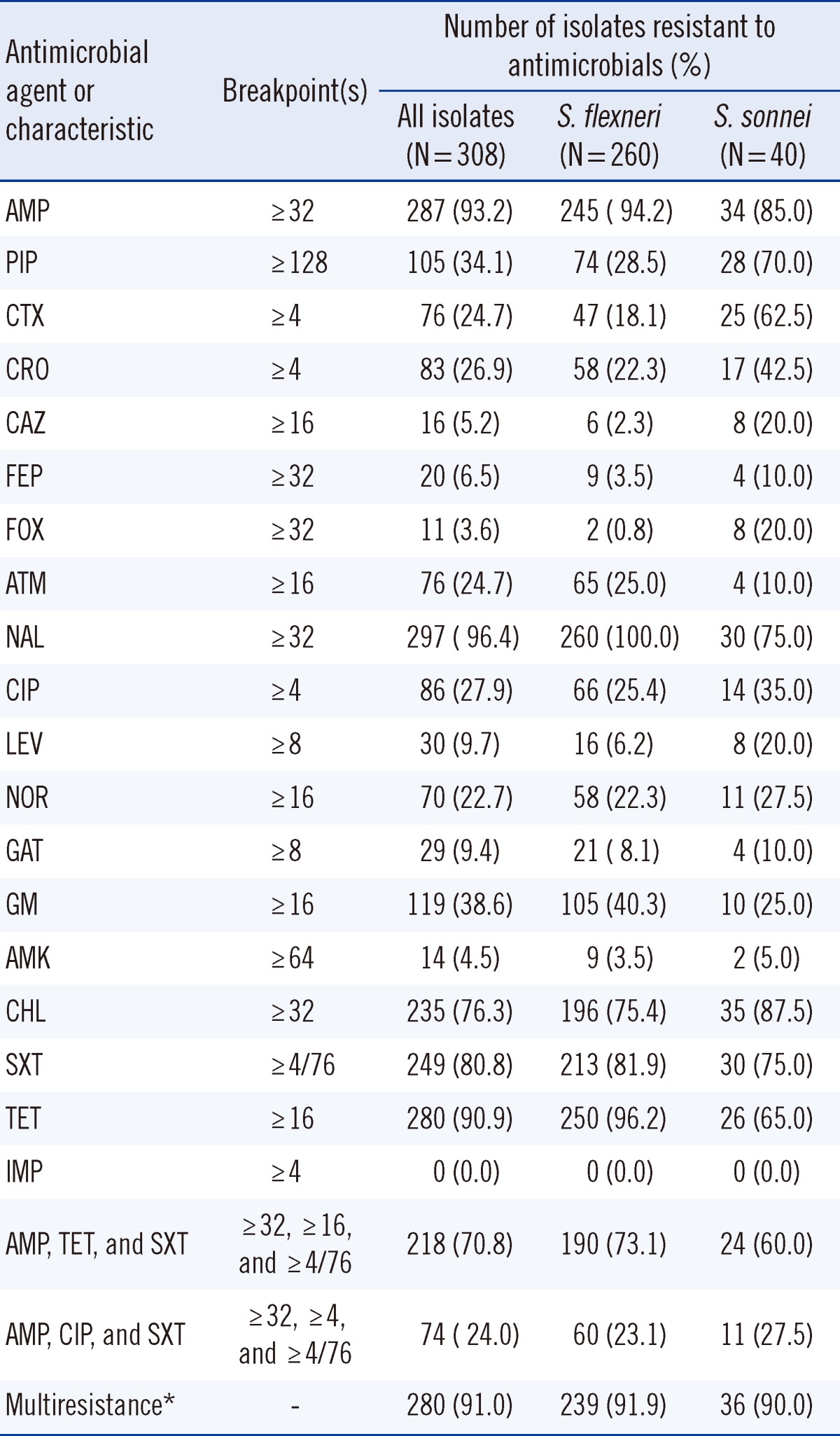
*Defined as resistant to 2 or more antimicrobial agents.
Abbreviations: AMP, ampicillin; PIP, piperacillin; CTX, cefotaxime; CRO, ceftriaxone; CAZ, ceftazidime; FEP, cefepime; FOX, cefoxitin; ATM, aztreonam; NAL, nalidixic acid; CIP, ciprofloxacin; LEV, levofloxacin; NOR, norfloxacin; GAT, gatifloxacin; GM, gentamicin; AMK, amikacin; CHL, chloramphenicol; SXT, trimethoprim/sulfamethoxazole; TET, tetracycline; IMP, imipenem.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download