Abstract
Here we report the cytogenetic and clinical manifestations observed in a patient with a rec(20)dup(20p)inv(20)(p11.2q13.3)mat. The patient was a full-term newborn girl with asymmetric intrauterine growth restriction and multiple congenital malformations, including a ventricular septal defect, pulmonary atresia, ambiguous genitalia, clinodactyly, and sacral dimpling. To our knowledge, this is the 4th report in the world and the 1st one in Korea of a patient with rec(20)dup(20p).
A recombinant chromosome is a structurally rearranged chromosome with a new segmental composition resulting from meiotic crossing-over between a displaced segment and its normally located counterpart in certain types of structural heterozygotes (most often inversion and insertion heterozygotes) [1]. This type of rearrangement can occur in any autosomal or sex chromosome; however, partial trisomy 20p and partial monosomy 20q resulting from meiotic recombination of chromosome 20 is very rare [2-5].
Here we report a female newborn with a rec(20)dup(20p) chromosome abnormality derived from recombination of a maternal chromosome 20 with pericentric inversion by meiotic crossing-over within the inversion loop. To our knowledge, this is the 4th report in the world and the 1st one in Korea of a patient with rec(20)dup(20p) [2-4].
A 30-yr-old woman in her first pregnancy had a routine fetal ultrasound at 23 weeks of gestation, and a fetal heart anomaly was suspected. Fetal echocardiography was performed, and a ventricular septal defect and pulmonary atresia were suspected. Her family history was unremarkable.
A female baby was born at 38+2 weeks' gestation. Her birth weight, height, and head circumference were 2,350 g (<3rd percentile), 47.5 cm (<50th percentile), and 34 cm (<40th percentile), respectively; these findings indicated asymmetric intrauterine growth restriction. Physical examination showed a heart murmur, ambiguous genitalia, sacral dimpling, and clinodactyly. On echocardiogram, there were ventricular septal defect, pulmonary atresia, patent ductus arteriosus, and pulmonary artery hypoplasia. Kidney, ureter, and bladder radiography and kidney ultrasound detected grade 2 left hydronephrosis. Spinal ultrasound was normal, and FISH analysis for DiGeorge syndrome (Vysis, Downers Grove, IL, USA) was negative (no 22q11.2 deletion). A chromosomal study of peripheral blood lymphocytes by G-banding identified an abnormal chromosome 20. Additional material of unknown origin was attached to band 20q13.3, and the initial karyotype was 46,XX,add(20)(q13.3). A parental chromosome study was recommended, and the examination revealed a normal paternal karyotype (46,XY) and an abnormal maternal karyotype [46,XX,inv(20)(p11.2q13.3)]. Both parents had normal phenotypes. Because the mother had pericentric inversion of chromosome 20, the patient's karyotype was modified to 46,XX,rec(20)dup(20p)inv(20)(p11.2q13.3)mat, according to the International System for Human Cytogenetic Nomenclature (2009). This indicates that fertilization of an ovum carrying rec(20)dup(20p) by a karyotypically normal sperm resulted in trisomy for the segment 20pter to 20p11.2 and monosomy for the segment 20q13.3 to 20qter (Fig. 1).
She underwent a Rastelli operation to correct the cardiac defects at 8 months of age. She had psychomotor developmental delay at 17 months of age according to the Denver Developmental Screening Test. Her fine motor, gross motor, and language function clustered around the 5- to 6-month level, and personal-social development was at 3 to 4 months.
Classically, meiotic crossing-over in inversion heterozygotes occurs via the reversed loop model (Fig. 2). The configuration of the bivalent allows as good an alignment and pairing as possible between matching segments in the inversion chromosome and its normal homolog. A single cross-over within the inversion loop leads to the production of 2 complementary recombinant chromosomes; one has a duplication of the distal segment of the short arm and a deletion of the distal segment of the long arm, while the other has the reverse. Therefore, the resulting conceptus would either have partial trisomy of distal 20p and partial monosomy of distal 20q, or partial monosomy of distal 20p and partial trisomy of distal 20q.
Cases of partial trisomy 20p are rare. Most reported cases resulted from parental reciprocal translocation, while only a few cases arose from parental inversion. In our literature review, we found only 3 reports of patients born alive with pure partial trisomy 20p and partial monosomy 20q resulting from meiotic recombination due to parental pericentric inversion during gametogenesis [2-5]. We reviewed the clinical features of the 3 previously reported cases [2-4] and this case of rec(20)dup(20p) (Table 1).
The clinical features of trisomy 20p with no other chromosomal imbalance have not yet been clearly defined. The common clinical findings associated with partial trisomy 20p are mental retardation, delay of psychomotor development, normal growth pattern, round face with prominent cheeks, dental anomalies, vertebral anomalies, poor motor coordination, poor speech, occipital flattening, hypertelorism, epicanthic folds, strabismus, short nose with large nostrils, congenital heart defects, renal anomalies, and other anomalies of fingers and toes [6, 7]. The frequencies of cardiac, renal, and digital anomalies in trisomy 20p patients are 36%, 75%, and 92%, respectively [8]. Although our case has many similarities to previous reports of trisomy 20p, our patient differed in that she was born with ambiguous external genitalia. Genital abnormalities are not common in patients with pure trisomy 20p. Hypospadias and cryptorchidism have been previously reported in cases with translocated 20p to other chromosomes [9, 10]. Macroorchidism has been described in a patient with de novo trisomy 20p [11]. These genital abnormalities are induced by trisomy of nearly the entire short arm of chromosome 20. In these cases, the breakpoints on 20p were different from those in our case. In the cases of pure 20q subtelomeric deletion, the most consistently shared feature was developmental delay, while dysmorphic features were also common [4].
Since the same chromosomal abnormality due to the recombination can recur, and approximately half of their phenotypically normal offspring would be inversion heterozygotes, genetic counseling and prenatal diagnosis were recommended to the parents for future pregnancies. According to the French database Human Cytogenetics Forum (www.hc-forum.net), the recurrence risk for recombination of this inversion was estimated at approximately 33%. This recombination is dependent upon the probability of chiasma formation, which enables the chromatids to cross over within the inverted segment during meiosis.
Our case of a rec(20)dup(20p) chromosome abnormality derived from a maternal pericentric inversion might aid in the elucidation of genotype-phenotype correlations in patients with trisomy 20p. Cytogeneticists should be aware that parents with an inverted chromosome can pass on this peculiar type of chromosomal abnormality to their offspring via meiotic recombination, and they should also be able to express the correct recombinant karyotype using standard nomenclature.
Figures and Tables
Fig. 1
Idiograms showing (A), the maternal normal chromosome 20; (B), the maternal inv(20)(p11.2q13.3); and (C), the patient's rec(20)dup(20p) chromosome.
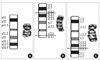
Fig. 2
Diagram of a possible meiotic arrangement of the inverted chromosome 20 and its normal homolog, showing crossing-over within the inversion loop. The arrows indicate the pericentric inversion breakpoints, and the cross indicates crossing-over within the inversion loop (For the sake of simplicity, only 2 of the 4 chromatids involved in the cross-over are depicted; the recombinant chromosomes shown at the bottom are generated from these 2 chromatids).

References
1. Shaffer LG, Slovak ML, editors. ISCN 2009 : An international System for Human Cytogenetic Nomenclature (2009). 2009. Basel: Karger.
2. Lucas J, Le Mée F, Le Marec B, Pluquailec K, Journel H, Picard F. Trisomy 20p derived from a maternal pericentric inversion and brachymesophalangy of the index finger. Ann Genet. 1985. 28:167–171.
3. Bown N, Cross I, Davison EV, Burn J. Partial trisomy 20p resulting from a recombination of a familial pericentric inversion. Hum Genet. 1986. 74:417–419.

4. Descipio C, Morrissette JD, Conlin LK, Clark D, Kaur M, Coplan J, et al. Two siblings with alternate unbalanced recombinants derived from a large cryptic maternal pericentric inversion of chromosome 20. Am J Med Genet. 2010. 152A:373–382.

5. Molina-Gomes D, Nebout V, Daikha-Dahmane F, Vialard F, Ville Y, Selva J. Partial trisomy 20p resulting from recombination of a maternal pericentric inversion: case report of a prenatal diagnosis by chorionic villus sampling. Prenat Diagn. 2006. 26:239–241.

6. Oppenheimer S, Dignan P, Soukup S. Partial trisomy 20p: familial occurrence. Am J Med Genet. 2000. 95:316–319.

7. Grammatico P, Cupilari F, Di Rosa C, Falcolini M, Porto GD. 20 p duplication as a result of parental translocation: familial case report and a contribution to the clinical delineation of the syndrome. Clin Genet. 1992. 41:285–289.

8. Sidwell RU, Pinson MP, Gibbons B, Byatt SA, Svennevik EC, Hastings RJ, et al. Pure trisomy 20p resulting from isochromosome formation and whole arm translocation. J Med Genet. 2000. 37:454–458.

9. Schinzel A. Trisomy 20pter to q11 in a malformed boy from a t(13;20) (p11;q11) translocation-carrier mother. Hum Genet. 1980. 53:169–172.

10. Taylor KM, Wolfinger HL, Brown MG, Chadwick DL, Franche U. Partial trisomy 20p derived from a t(18;20) translocation. Hum Genet. 1976. 34:155–162.

11. Balestrazzi P, Virdis R, Frassi C, Negri V, Rigoli E, Bernasconi S. "De Novo" trisomy 20p with macroorchidism in a prepuberal boy. Ann Genet. 1984. 27:58–59.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download