Abstract
The -D- phenotype is a rare Rh phenotype that strongly expresses D antigen without C, c, E, or e antigens. In -D- phenotype individuals, anti-Rh17 (Hro) is commonly found if there is a history of pregnancy or transfusion with red blood cells (RBCs) that express C, c, E, or e antigens. We report the first case of a -D- phenotype patient with multiple Rh antibodies including anti-Rh17 who had a history of two occasions of transfusion with eight random donor platelet concentrates two and six years ago. We found that a trivial amount of RBCs in the platelet components was able to trigger sensitization to RBC antigens, especially the highly immunogenic and clinically significant Rh antigens, including C, c, E, e or CcEe polypeptides. To avoid unnecessary sensitization and to minimize the risk of hemolytic transfusion reactions in patients with this rare Rh phenotype, a modified strategy for pretransfusion screenings needs to be discussed in the field of transfusion medicine.
-D- is a rare Rh phenotype in which D antigen is strongly expressed but C, c, E, or e antigens are not. This blood type is a consequence of a deletion or recombination mutation of the RHCE gene, which encodes for the C, c, E, and e antigens. Okubo et al. found 7 -D- individuals (0.001%) among 692,000 Japanese blood donors and estimated the frequency of the -D- haplotype as 0.0032 among Japanese [1]. With the exception of 3 cases reported since the initial case report in 1998, there is no data about the prevalence of -D- phenotype in Korea [2-6]. The significance of the -D- phenotype is that individuals who have it can make multiple Rh antibodies against C, c, E, or e antigens if they are sensitized to Rh antigens, and this puts them at risk of massive hemolytic transfusion reactions. Thus far, the clinical relevance of the -D- phenotype has been predominantly reported in pregnant women, causing a mild to fatal hemolytic disease of the fetus and newborn [2-7]. Nevertheless, it is generally not indicated to transfuse C, c, E, or e antigen-positive red blood cells (RBCs) to a -D- phenotype patient. Here we report a case in which a -D- phenotype male patient might have been sensitized to Rh antigens resulting from the transfusion of random donor platelet concentrates and showed presence of multiple Rh antibodies, including anti-Rh17. Two other family members having the -D- phenotype were not sensitized even through multiple pregnancies.
A 49-yr-old Korean male with chronic hepatitis B and liver cirrhosis visited the emergency room with esophageal variceal bleeding. His hemoglobin level was 8.4 g/dL. Other laboratory findings were as follows: white blood cell count, 3,090/µL; platelet count, 40,000/µL; total protein level, 5.2 g/dL; albumin level, 3.1 g/dL; AST/ALT, 67/55 U/L; prothrombin time (PT), 16.8 sec; and activated partial thromboplastin time (aPTT), 34.3 sec. RBC transfusion was considered, and a type and screen test was requested. His blood group was A and RhD+, and importantly, a strong agglutination reaction with anti-D sera was readily observed. In an antibody screening test, the serum of the patient agglutinated with all screening panel cells. The results from antibody identification tests using commercially available kits, ID-System (Bio-Rad, Philadelphia, PA, USA) and Resolve Panel A (Ortho-Clinical Diagnostics Company, Raritan, NJ, USA) were inconclusive because the serum of the patient reacted strongly and agglutination was observed in both panel cells. Each of the reactions was 4+ macroscopic in 37℃ phase and in the anti-human globulin phase.
We performed Rh phenotyping with DiaMed-ID (Bio-Rad, Philadelphia, PA, USA) and found that the D antigen was present, but C, c, E, and e antigens were not expressed on the RBCs of the patient. Further Rh antibody identification tests were performed using an in-house panel of 8 donor cells with ID-IAT and ID-Papain systems (DiaMed, Bio-Rad Laboratories, Cressier sur Morat, Switzerland) at the Central Laboratory of the Swiss Red Cross in Bern. Multiple Rh antibodies, including anti-Rh17, anti-e, and anti-Ce, were identified in serum of the patient. Based on these collective findings, we interpreted these results as being positive for the -D- phenotype. The patient did not have a previous history of RBC transfusion. However, he had received eight units of random donor platelet concentrates, one was given two years ago and the other given 6 yr ago. No transfusion-related problems were noted at the either time. The antibody screening test, which had been performed just before the first platelet transfusion, was negative. Through the extensive review of the past medical history of the patient and by directly interviewing him, we could not isolate any specific episode other than the platelet transfusions that we could reasonably suspect of inducing Rh antigen sensitization in the patient. After esophageal variceal ligation therapy, his hemoglobin level gradually increased to over 10 g/dL for 2 weeks without transfusion.
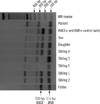
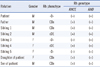
Since the -D- phenotype is derived from a homologous deletion of the RHCE genes and is passed on to descendants in a Mendelian ratio, Rh phenotyping and genotyping tests were performed on each family member including his biological father, siblings, and children, after obtaining informed consent (Table 1). For RHD/RHCE genotyping, allele-specific polymerase chain reactions (AS-PCR) for intron 4 of the RHD and RHCE genes were performed using a method and primers as previously described [8, 9]. Healthy, control individuals possess both the 115 bp and 230 bp bands, which represent the presence of RHD and RHCE genes, respectively (Fig. 1). However, only the 115 bp product was amplified in this patient and his 2 siblings (Table 1, Sibling 3 and 5), which confirmed the deletion of the RHCE gene.
The genotype of deceased mother of the patient was presumed to be -D-/cDE according to the pedigree analysis. Both the elder sister and younger sister of patient, identified as -D- phenotype (Table 1, Sibling 3 and 5), have 2 children with similar Rh phenotypes. However, both sisters of the patient did not have any detectable Rh antibodies. They did not have a history of either blood transfusion or abortion. Underlying medical conditions of the elder sister (Table 1, Sibling 3) were well-controlled diabetes mellitus and essential hypertension, and the younger sister (Table 1, Sibling 5) was a carrier of the hepatitis B virus. For future testing of -D- RBCs in the family members of the patient, -D- RBCs were collected at 3 separate times from the patient and his elder sister. Three units of -D- RBCs from each were deposited in the blood bank of Samsung Medical Center after being frozen with a cryoprotective agent Glycerolyte 57 (Fenwal Laboratories, Chicago, IL, USA), following the protocol of the New York Blood Center [10].
RhD typing is usually included in pre-transfusion screening tests and routine prenatal check-ups. However, Rh phenotyping for C, c, E, and e antigens is not frequently performed. For this reason, the -D- phenotype is rarely detected during pre-transfusion screenings. The transfusion of blood components having different Rh antigens except D is performed without specific consideration given to the -D- phenotype, and it is presumed that the antibody screening test would be negative and the cross-match is compatible. The -D- phenotype may be suspected in cases with stronger agglutination reactions in RhD typing, which is now understood to be a consequence of the insertion of RHD into RHCE in tandem with the RHD gene [11], but it is likely to be neglected due to variable reactivity.
Apparently, the 2 female siblings (Table 1, Sibling 3 and 5) with the -D- phenotype were not sensitized by pregnancies, this observation was in contrast to the several reported cases of -D- women who had been sensitized through pregnancy [2, 7]. As for the patient, the only presumptive cause of the sensitization was the previous transfusions with random donor platelet concentrates that likely contained a substantial number of C, c, E, or e positive RBCs. These particular RBCs likely enabled this sensitization. It is shown that 1 unit of platelet concentrate contains about 2.2-2.3×104/µL of RBCs according to the data from Korean Red Cross Blood Center [12].
In most cases, the factors that trigger sensitization in -D- individuals are known to be pregnancy or the transfusion of packed RBCs [2-6, 13-17]. To the best of our knowledge, there has been no reported case of a -D- individual sensitized by platelet transfusion. It seems that transfusion with any blood component contaminated by RBCs is able to efficiently trigger the sensitization to C, c, E, e or CcEe polypeptide antigens in a similar way as the D antigen, which is reported to trigger the formation of anti-D with only 0.5-1.0 mL of transfused RBCs [18, 19].
In summary, in a patient with a rare Rh phenotype, -D- and multiple Rh antibodies were found in type and screen test for transfusion. The only presumptive cause of this sensitization was a history of transfusion with random donor platelet concentrates. This strongly suggests that trivial amounts of RBCs in platelet concentrates are able to trigger sensitization to the highly immunogenic C, c, E, or e antigens and induce the formation of clinically significant anti-Rh17 and other Rh antibodies. Therefore, we suggest that Rh phenotyping and antibody screening be performed in pre-transfusion screenings for any cellular blood component in order to detect individuals with this rare Rh phenotype. This will be helpful to prevent sensitization and to minimize the risk of hemolytic transfusion reactions.
Figures and Tables
Acknowledgement
We thank Dr. Hyun Ok Kim, Department of Laboratory Medicine, Yonsei University College of Medicine for her assistance in the preparation of frozen glycerolized RBCs. We also thank Dr. Stefano Fontana and Hein Hustinx at the Central Laboratory of the Swiss Red Cross in Bern, Switzerland for identification of the Rh antibodies.
References
1. Okubo Y, editor. Pretransfusion testing with special reference to blood groups (in Japanese). 1991. Tokyo: Ishiyaku Publishers, Inc.;40–41.
2. Han KS, Kim HC, Shim WS. A case of fatal hemolytic disease of the newborn associated with-D-/-D-phenotype. Am J Perinatol. 1997. 14:495–497.

3. Whang DH, Kim HC, Hur M, Choi JH, Park JS, Han KS. A successful delivery of a baby from a D--/D-- mother with strong anti- Hr0. Immunohematology. 2000. 16:112–114.
4. Hwang JY, Suh IB, Shim JY, Cho JS, Lee DH. A case of fetal hydrops associated with maternal -D-/-D- phenotype. Korean J Obstet Gynecol. 2006. 49:1378–1382.
5. Um TH, Cho CR, Whang JH, Whang DH, Yoon MS, Han KS. A case of D--/D-- phenotype associated with moderate hemolytic disease of the newborn. Korean J Blood Transfus. 2007. 18:61–65.
6. Han KS, Kim HC, Shim WS, Yoon MS, Joo KW, Hahn KS, et al. A family with -D- phenotype associated with fatal hemolytic disease of the newborn. Korean J Blood Transfus. 1995. 6:201–206.
7. Mertens G, Muylle L, Ursi JP, Docx M, Renard JM. Transfusion with thawed Rh -- D blood of a newborn suffering from haemolytic disease due to anti-Rh 17. J Obstet Gynaecol. 1997. 17:590–591.
8. Huang CH. Alteration of RH gene structure and expression in human dCCee and DCW-red blood cells: phenotypic homozygosity versus genotypic heterozygosity. Blood. 1996. 88:2326–2333.
9. Denomme GA, Rios M, editors. Molecular protocols in transfusion medicine. 2000. San Diego: Academic press;85–93.
10. Choi KH, Rhu JH, Park HR, Kim HO. The preparation of frozen red blood cells and a procedure for deglycerolizing frozen RBCs using COBE 2991 blood cell processor. Korean J Blood Transfus. 2001. 12:189–196.
12. Kim CA, Seo DH, Hwang BG, Kim SI. Current status of the quality control of blood components at the Korean Red Cross blood centers. J Clin Pathol Qual Control. 2001. 23:319–323.
13. De Vooght KM, Demir AY, Folman CC, Schutgens RE, van Solinge WW, Kemperman H. Successful transfusion care for a patient with the Rhesus -D- phenotype and antibodies against Rh17 and two additional alloantibodies. Ann Hematol. 2012. 91:963–964.

14. Aref K, Boctor FN, Pande S, Uehlinger J, Manning F, Eglowstein M, et al. Successful perinatal management of hydrops fetalis due to hemolytic disease associated with D-- maternal phenotype. J Perinatol. 2002. 22:667–668.

15. Brumit MC, Carnahan GE, Stubbs JR, Storry JR, Reid ME. Moderate hemolytic disease of the newborn (HDN) due to anti-Rh17 produced by a black female with an e variant phenotype. Immunohematology. 2002. 18:40–42.

16. Deitenbeck R, Tutschek B, Crombach G, Stannigel H. Successful management of pregnancy and hemolytic disease of the newborn due to anti-HrO in a woman of the D--phenotype. Transfusion. 1999. 39:1151–1152.
17. Hirose M, Nakanishi K, Kaku S, Moro H, Hodohara K, Aotani H, et al. Fetal hemolytic disease due to anti-Rh17 alloimmunization. Fetal Diagn Ther. 2004. 19:182–186.

18. Urbaniak SJ. A successful program of immunizing Rh-negative male volunteers for anti-D production using frozen/thawed blood. Transfusion. 1981. 21:64–69.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download