Abstract
The in vitro antifungal susceptibility of 636 Candida bloodstream isolates collected from 15 tertiary hospitals in Korea was determined using the Vitek-2 yeast susceptibility system (bioMérieux, France). Overall susceptibility rates were 98.1%, 95.9%, 99.1%, and 97.3% for amphotericin B, fluconazole, voriconazole, and flucytosine, respectively. The results show that the rates of resistance to 4 antifungal drugs remain low among Candida bloodstream isolates in Korea.
The Vitek-2 yeast susceptibility system (bioMérieux, Marcy l'Étoile, France) is a fully automated commercial method that allows determination of the minimal inhibitory concentration (MIC) of 4 antifungal agents, i.e., amphotericin B, fluconazole, voriconazole, and flucytosine [1, 2]. This system has demonstrated a high level of reproducibility and has an excellent categorical agreement with the CLSI microdilution reference method [1-3]. Moreover, a recent study showed that the Vitek-2 system is superior to the CLSI or the European Committee on Antimicrobial Susceptibility Testing (EUCAST) broth microdilution method for detecting amphotericin B-resistant Candida isolates [4]. Because of its advantages, including a significant reduction in technologist hands-on time and turnaround time [1, 2], this system has become one of the most widely used antifungal susceptibility testing systems in Korean clinical laboratories. However, nationwide data for the in vitro antifungal susceptibility of Candida bloodstream infection (BSI) isolates as determined by the Vitek-2 system are still lacking in Korea. In this study, we investigated the in vitro activity of 4 antifungal agents using the Vitek-2 system against Candida BSI isolates recovered from 15 tertiary hospitals in Korea.
During the study period (September 2007 to August 2008), 636 Candida BSI isolates were collected from 636 patients among the 15 tertiary hospitals in Korea. Antifungal susceptibility testing with the Vitek-2 system was performed according to the manufacturer's instructions [1, 2]. The categorical result was obtained according to the breakpoints provided by the Vitek-2 system for amphotericin B (susceptible [S], ≤1 µg/mL; intermediate, 2 µg/mL; resistant [R], ≥4 µg/mL), fluconazole (S, ≤8 µg/mL; susceptible dose dependence [SDD], 16 to 32 µg/mL; R, ≥64 µg/mL), and voriconazole (S, ≤1 µg/mL; SDD, 2 µg/mL; R, ≥4 µg/mL), and flucytosine (S, ≤4 µg/mL; intermediate, 8-16 µg/mL; R, ≥32 µg/mL).
Table 1 summarizes the in vitro antifungal susceptibilities of the 636 Candida BSI isolates to 4 antifungal agents as determined by using the Vitek-2 system. For all 636 Candida BSI isolates combined, the activity of each agent (µg/mL), expressed as the MIC50/MIC90 (and the percentage of susceptible isolates) was as follows: amphotericin, 0.5/0.5 (98.1%); fluconazole, ≤1/2 (95.9%); voriconazole, ≤0.12/≤0.12 (99.1%); and flucytosine, ≤1/≤1 (97.3%). These results represent the updated nationwide data, which show that the majority of Candida BSI isolates was susceptible to all 4 fungal agents tested.
In our previous multicenter study, nearly all isolates (99.7%) had a MIC ≤1 µg/mL for amphotericin B [5]. In the present study, resistance to amphotericin B was found in 5 Candida isolates; whereas intermediate resistance was found in 7 isolates. These Vitek-2 results produced no major errors when the E test was used as reference standard, supporting our previous report that Vitek-2 is more sensitive for detecting amphotericin B resistance among Candida species than the CLSI method [4].
According to the SENTRY Antimicrobial Surveillance Program [6], the percentages of Candida BSI isolates with resistance to fluconazole and voriconazole are 2.5% and 1.2%, respectively. In the present study, resistance to fluconazole was found in 1.4% (9/636) of the Candida isolates (4 C. krusei, 3 C. glabrata, and 2 C. parapsilosis) and resistance to voriconazole was found in 0.9% (6/636) of the Candida isolates (1 C. albicans and 5 C. glabrata), which was comparable with our previous report [5].
Until now, nationwide data on the in vitro antifungal activity of flucytosine against Candida BSI isolates were not available in Korea. Results from the global SENTRY Antimicrobial Surveillance Program (2008) showed that 95.5% of the 1,201 Candida BSI isolates from 5 continents are susceptible to flucytosine [6]. Resistance to flucytosine was noted in 2.4% of C. albicans, 0% of C. glabrata, 0.5% of C. parapsilosis, and 10.3% of C. tropicalis isolates [6]. In this study, we showed, for the first time, that 97.3% of Candida BSI isolates from Korea were susceptible to flucytosine, as determined by using the Vitek-2 system. Only 3.2% of C. albicans, 1.5% of C. parapsilosis, 1.4% of C. glabrata, and 0% of C. tropicalis isolates were resistant to this agent. Again, our results show that the low rates of resistance to flucytosine are consistent with reports from other countries.
Figures and Tables
Table 1
In vitro antifungal susceptibilities of 636 isolates of Candida species to fluconazole, voriconazole, amphotericin B, and flucytosine, as determined by using the Vitek-2 system
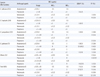
*SDD, I and R (susceptible-dose dependent, intermediate and resistant, respectively), using the Vitek-2 interpretive breakpoint criteria; †Includes Candida guilliermondii (10 isolates), C. famata (6 isolates), C. krusei (4 isolates), C. pelliculosa (4 isolates), C. utilis (3 isolates), C. pseudohaemulonii (2 isolates), C. lusitaniae (1 isolate), and C. intermedia (1 isolate); ‡All 4 C. krusei isolates are considered to be resistant to fluconazole, irrespective of the minimum inhibitory concentration (MIC).
Acknowledgement
This study was supported by a grant (CRI09054-1) from the Chonnam National University Hospital Research Institute of Clinical Medicine, and the Basic Science Research Program, through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0021556).
References
1. Pfaller MA, Diekema DJ, Procop GW, Rinaldi MG. Multicenter comparison of the VITEK 2 antifungal susceptibility test with the CLSI broth microdilution reference method for testing amphotericin B, flucytosine, and voriconazole against Candida spp. J Clin Microbiol. 2007. 45:3522–3528.
2. Pfaller MA, Diekema DJ, Procop GW, Rinaldi MG. Multicenter comparison of the VITEK 2 yeast susceptibility test with the CLSI broth microdilution reference method for testing fluconazole against Candida spp. J Clin Microbiol. 2007. 45:796–802.
3. Bourgeois N, Dehandschoewercker L, Bertout S, Bousquet PJ, Rispail P, Lachaud L. Antifungal susceptibility of 205 Candida spp. isolated primarily during invasive candidiasis and comparison of the Vitek 2 system with the CLSI broth microdilution and Etest methods. J Clin Microbiol. 2010. 48:154–161.
4. Shin JH, Kim MN, Jang SJ, Ju MY, Kim SH, Shin MG, et al. Detection of amphotericin B resistance in Candida haemulonii and closely-related species using the Etest, Vitek-2 Yeast Susceptibility System, and CLSI and EUCAST broth microdilution methods. J Clin Microbiol. 2012. 50:1852–1855.
5. Jung SI, Shin JH, Song JH, Peck KR, Lee K, Kim MN, et al. Multicenter surveillance of species distribution and antifungal susceptibilities of Candida bloodstream isolates in South Korea. Med Mycol. 2010. 48:669–674.
6. Messer SA, Jones RN, Moet GJ, Kirby JT, Castanheira M. Potency of anidulafungin compared to nine other antifungal agents tested against Candida spp., Cryptococcus spp., and Aspergillus spp.: results from the global SENTRY Antimicrobial Surveillance Program (2008). J Clin Microbiol. 2010. 48:2984–2987.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download