Abstract
Background
This study was aimed to investigate the genetic diversity and antibiotic resistance profile of the nosocomial infection agent Acinetobacter baumannii from a medical intensive care unit (ICU) in a teaching hospital in Suzhou, China.
Methods
The genetic relationship among A. baumannii isolates in an ICU was investigated using multilocus sequence typing (MLST). The antibiotic resistance pattern was determined by performing an antibiotic susceptible test, which included an agar dilution method and an E-test method. Resistant determinants, e.g., carbapenemase genes, metallo-β-lactamases, and class 1 integron, were analyzed by specific PCR and DNA sequencing.
Results
In the present study, 33 non-duplicate isolates were identified as 5 existing sequence types (STs) (ST92, ST75, ST112, ST145, and ST345) and 1 new sequence type STn, which has a G-A mutation at nt268 on ropD40 of ST251. These results reveal limited diversity in carbapenem non-susceptible A. baumannii (CNSAb) isolates in our ICU, which are comprised of only 2 distinct STs, with ST92 and ST75 clustering into a clonal complex (CC) 92. Most CNSAb isolates (94.4%, 17/18) harbored the OXA-23 gene, while no carbapenem-susceptible A. baumannii (CSAb) isolates harbored it. In addition, 66.7% (22/33) isolates were positive for class 1 integrase, and gene cassette analysis showed there are 3 gene arrays among them, i.e., aacA4-catB8-aadA1 (77.3%, 17/22), aacA4 (22.7%, 5/22), and aacC1-orfX-orfX'-aadA1 (4.5%, 1/22).
Acinetobacter baumannii is an opportunistic human pathogen associated with an increasing incidence of nosocomial infections, including bacteremia, urinary tract infections, and ventilator-associated pneumonia [1-3]. Most A. baumannii isolates have a multidrug resistant phenotype (MDRAb) with an increasing prevalence in the intensive care unit (ICU) [2, 4]. Although carbapenem is a common clinical choice to treat MDRAb infections, the resistance rate has increased dramatically over the past decade. Carbapenem resistance is caused by the production of carbapenemases, including class D β-lactamases (OXA-type carbapenemases) and class B metallo-β-lactamases (MBLs) as previously described [5-8]. The OXA-type carbapenemases are comprised of 4 kinds of genes: blaOXA-23-like, blaOXA-24-like, blaOXA-58-like, and an intrinsic blaOXA-51-like type as previously reported [9, 10]. In addition, ISAba1, an insertion sequence (IS), was found in A. baumannii isolates, which can enhance the expression of OXA-type carbapenemase genes and mobilize these genes among the strains [11].
Integron is a widely studied vehicle for gene capture, which is highly related to antibiotic resistant gene dissemination [12, 13]. Among MDRAb isolates, the class 1 integron is commonly detected, which infers a high association with carbapenem resistance. The integron structure contains an integrase, followed by an attI site for the integration of cassettes and the recognition of integrase, and a promoter to drive its expression. It can appear on a plasmid or chromosome, and can be classified into different types (class 1, class 2, and class 3 integron) on the basis of their integrases. The most classical integron, class 1 integron, has 2 conserved terminal sequences. Therefore, the primer set (5'-conserved sequence [CS] and 3'-CS), which targets 2 terminal sequences in the integron, is commonly used for integron cassette analysis.
Multi locus sequence typing (MLST) is an unambiguous typing method for identifying accurate and portable nucleotide sequences of internal fragments of multiple housekeeping genes. Recently, some studies [14-16] revealed that the clonal complex (CC) 92 in the carbapenem non-susceptible A. baumannii (CNSAb) isolate has a worldwide dissemination. The ST92 A. baumannii isolate is the largest clone and is predicted to be the founder of this clonal complex. Molecular epidemiological surveillance not only provides a better understanding of nosocomial infections in certain hospitals, but also provides a chance to understand the route of global dissemination. However, the genetic diversity and molecular characteristics of A. baumannii in our region remain unclear. Therefore, in order to better understand the epidemiological characteristics of A. baumannii in the ICU and the relationship of carbapenem resistance with integrons, in this study, we performed MLST typing and a molecular investigation of antibiotic resistance determinants in A. baumannii isolates collected in our medical ICU.
In total, 33 non-duplicated clinical A. baumannii isolates were collected by standard isolation methods between January and December 2009 in our medical ICU. All isolates were identified by conventional methods and further identified by 16S-23S rRNA gene spacer region analysis as described previously [17]. All of these isolates were stored at -80℃ in brain-heart infusion broth containing 50% (v/v) glycerol before inclusion in this study.
Antimicrobial susceptibility of isolates to cefepime, ceftazidime, piperacillin, amikacin, gentamicin, ciprofloxacin, ampicillin/sulbactam, and piperacillin/tazobactam were determined by using the disc diffusion method. The minimum inhibitory concentrations (MICs) of imipenem and meropenem were determined by using the agar dilution method, and that of colistin was determined by using the E-test (AB bioMérieux, Marcy-l'Etoile, France). Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as reference strains for the antimicrobial susceptibility test. The results were interpreted according to the manufacturer's instructions and CLSI guidelines [18].
Genomic DNA from A. baumannii was extracted using a genomic DNA extraction Kit (Qiagen, Valencia, CA, USA) following the manufacturer's instructions. MBL-producing isolates were screened using the imipenem-EDTA double-disk synergy test [19]. Metallo-β-lactamase (IPM4)-producing A. baumannii was used as a positive control. The genes encoding carbapenemases blaIMP-type, blaVIM-type, blaSIM-1, blaOXA-23-like, blaOXA-24-like, blaOXA-58-like, and blaOXA-51-like were analyzed by PCR using primers as described previously [5-8]. The location of blaOXA-23-like and blaOXA-51-like with an insert element (ISAba1) was screened by PCR [11].
Integron analysis was conducted by using PCR with the class 1 integrase (int1 gene) as the targeting gene, as previously described [12]. Gene cassettes in class 1 integrase-positive isolates were performed by amplifying the sequence from the 5'-CS to the 3'-CS region [12]. The PCR products were purified and sequenced, either directly by using the PCR product, or by cloning into a pGEM®-T vector (Promega, Madison, WI, USA). All sequencing reactions were performed using an ABI prism sequencer 3730 (Applied Biosystems, Foster City, CA, USA), and the sequence data were analyzed by using BLAST at the NCBI website (http://www.ncbi.nlm.nih.gov).
MLST was performed on A. baumannii as described by Bartual et al. [20]. Briefly, 7 housekeeping genes, i.e., gltA, gyrB, gdhB, recA, cpn60, gpi, and rpoD, were amplified, followed by sequencing on the ABI prism sequencer 3730 (Applied Biosystems). The sequences of these 7 housekeeping genes were analyzed using the Pubmlst database (http://pubmlst.org/abaumannii/). The sequence type (ST) was designated according to the allelic profiles in the database. The eBURST algorithm (version 3; http://eburst.mlst.net/) was used to assign STs to CCs and to assess the genetic relationship with definition of the groups sharing alleles at ≥6 of 7 loci [21]. The CC comprises a founding ST as a common ancestor and several other closely related STs descending from the predicted founding genotype.
Resistance rates for each antibiotic were compared between CNSAb and carbapenem-susceptible A. baumannii (CSAb), ST92-CNSAb and ST92-CSAb, and the int1-positive group and int1-negative group, using Fisher's exact test. A P value <0.05 was considered as significant difference.
In this study, 33 non-duplicate A. baumannii isolates were isolated from patients hospitalized in our medical ICU. All of these isolates were confirmed by 16S-23S rRNA internal spacer analysis as previously described [17]. Among these isolates (26 from lower respiratory tract, 4 from urinary tract, and 2 from blood, and 1 from pus), 54.5% (18/33) isolates were resistant to either imipenem, meropenem, or both. The resistance rate of colistin was 3% (1/33), which coincides with the observation that colistin is the most effective antibiotic to treat A. baumannii infections in our area. From a nationwide surveillance conducted during 2007, the resistance rates of imipenem and meropenem in China were calculated to be 40% and 35%, respectively [22]. However, from a new surveillance report generated in 2010, the prevalence of CNSAb isolates was dramatically increased to 80% [23]. Therefore, the CNSAb isolate is becoming a major concern as a nosocomial infection in China.
According to the MLST, 33 A. baumannii isolates can be grouped into 5 existing STs (ST92, 1-3-3-2-2-7-3; ST75, 1-3-3-2-2-11-3; ST112, 1-12-56-36-4-61-26; ST145, 21-35-2-28-1-52-4; ST345, 46-12-110-1-16-141-50) and 1 new ST, which has a G-A mutation at nt268 on the rpoD40 loci of ST251(32-48-54-35-1-11-40) (Table 1). All CNSAb isolates comprised of ST92 and ST75 only, accounting for 77.8% (14/18) and 22.2% (4/18), respectively.
Clonal relation analysis showed that both ST92 and ST75 belong to the CC92, which is a globally disseminated CC for A. baumannii (Fig. 1). This result suggests that CC92 is the major clone that spreads in the ICU of our hospital. eBURST analysis [21] showed that ST92 is the founder of CC92. Previous studies [14, 15, 24] showed that several STs exist, e.g., ST75, ST88, ST90, ST136, ST137, and ST138, which are SLVs of the founder ST92. However, we still found other types, e.g., ST381 and ST375, which are double locus variants (DLVs) of ST92.
No blaIMP-type, blaVIM-type, blaSIM-1, blaOXA-24-like, and blaOXA-58-like genes were observed among these isolates, and no MBLs producing these isolates were detected by the EDTA double-disk synergy test. Most (94.4%, 17/18) ST92-CNSAb isolates harbor the blaOXA-23 gene, as shown in Table 2. Interestingly, there was 1 ST92-CSAb isolate that could be detected as being positive for the blaOXA-23 gene. However, ISAba1 blaOXA-23 gene mapping showed that no ISAba1 gene could be detected in this isolate. Therefore, this result confirmed the importance of the ISAba1 gene in A. baumannii isolates, which exhibit the carbapenem resistance phenotype. ISAba1 was detected in most (88.2%, 15/17) blaOXA-23-positive isolates, which are located upstream of blaOXA-23. The blaOXA-51-like gene was detected in all isolates in this study, which is consistent with the blaOXA-51-like gene as an intrinsic carbapenemase. However, there were no ISAba1 genes located with the blaOXA-51-like gene as determined by PCR mapping in CSAb isolates. All blaOXA-51-like genes were confirmed as blaOXA-66 genes by sequencing of CNSAb isolates.
Integron analysis revealed that all CNSAb isolates have a class 1 integrase (Table 2). When comparing the antibiotic resistance profiles (Table 1), most antibiotics showed resistance rates that were much higher in the int1-positive isolates than in the negative ones. Integron gene cassette analysis showed that there were 3 distinct antibiotic gene arrays, i.e., aacA4-catB8-aadA1 (77.3%, 17/22; Genebank accession no. EU340419), aacA4 (22.7%, 5/22; Genebank accession no. EU523056), and aacC1-orfX-orfX'-aadA1 (4.5%, 1/22; Genebank accession no. AY307113). Functional analysis showed that these genes were only associated with aminoglycoside and chloramphenicol resistance, which are not related to carbapenem resistance. It seems that carbapenem-resistant genes might not be transmitted via integron structures in our area.
In this study, we analyzed the molecular typing and characteristics of A. baumannii isolates in our ICU. From the MLST result, the predominant clone is ST92 in CNSAb isolates in our hospital, and this observation is also consistent with previous studies [14-16, 25], which revealed that ST92 has spread worldwide. However, limited diversity was observed in CNSAb isolates in our ICU, while there was a higher diversity in CSAb isolates. Interestingly, there were still 8 CSAb isolates being assigned as ST92, but none of the CSAb isolates belonged to ST75. This characteristic indicates that these 2 types might exhibit differences in clinical outcomes or have antibiotic resistance patterns. Although there were no significant differences in antibiotic resistance rates for the ST92 and ST75 CNSAb isolates, they still exhibited slight differences on their MIC ranges. As shown in Table 2, all ST92 CNSAb isolates were resistant to imipenem, with MICs ranging from 32-128 mg/L; most of them (71.4%, 10/14) were also resistant to meropenem with the same MIC range. However, the MIC of the imipenem-resistant ST75 isolates ranged from 64 to 128 mg/L, which is a more narrow resistance profile than that of ST92 (Table 2). However, there was no difference in the MIC range of meropenem for these 2 types. These results indicate that ST75 might have a more severe imipenem resistance range than ST92.
Genetic analysis showed that ST75 is the single locus variant (SLV) of ST92, which differs in its gpi loci. While ST92 isolates show moderate carbapenem resistance rates, all ST75 types are resistant to carbapenem with a higher MIC range. Therefore, we hypothesized that mutations of the gpi loci in ST92, e.g., ST75, might benefit its survival when encountering carbapenem selection. In contrast, in our collection, there were some ST92-type CSAb isolates, but none was ST75, which also inferred that ST75 CNSAb isolates might represent a severe epidemic marker in nosocomial infections in our area. Moreover, a study by He et al. [15] showed that more ST138-CNSAb isolates are associated with ST92 in western China, which could give rise to the interesting assumption that the composition of CNSAb STs could exhibit geographic differences. These differences might be caused by different antibiotic usage habits, which may influence the evolution direction of ST92. Therefore, ST75 accompanied by ST92 might be the epidemical feature in eastern China.
In this study, we observed more diversity in CSAb isolates (5 ST types) than in CNSAb isolates (only 2 ST types). Interestingly, we observed some of CSAb isolates is ST92-CSAb type, and this type strain is the majority component in the epidemic CNSAb clonal CC92. While the other 4 STs, including ST112, ST145, ST345, and STn are singleton in the A. baumannii population, which shows no relation with epidemic strain CC92. As is known, natural selection is the major force that shapes the population structure of microbial communities in certain environments. A higher diversity in CSAb isolates might be due to fewer encounters with natural selection forces, e.g., antibiotics. Antibiotic resistance profiles showed that most antibiotics caused more resistance in the ST92-CNSAb group than in the ST92-CSAb group (Table 1). However, ST92-CSAb strains could potentially be a source of CNSAb strains, having acquired carbapenem resistance. Therefore, ST92-CSAb strains should be considered as important along with CNSAb isolates for future nosocomial infection control.
Genetic determinants analysis shows MBLs are not the major resistant determinants in CNSAb isolates in our hosptial, which is consistent with previous study [26]. However, in some distinct areas of China, the MBL plays a more important role than the OXA-type carbapenemase in CNSAb [27]. This implies that a geographical characteristic plays an important role in CNSAb isolates formation, and it could be due to different antibiotic usage in distinct areas. Integron analysis shows a high prevalence of this gene capture machinery. Although in this study, we only recovered 3 type of class 1 integron cassette, there still shows integron is highly associated with antibiotic resistance. However, there are some limitations in this integron cassette analysis method, e.g., some gene cassettes failed to be recovered due to the lack of a conserved terminal [28]. However, there are several studies [29, 30] showing that there are multiple gene cassettes that contain the OXA gene in A. baumannii isolates. This might indicate that transmission of the carbapenemase resistant gene might be mediated by a very complex mechanism. Notably, whole genome sequencing of MDRAbs [31-34] revealed a correlation between carbapenem resistance and the integron component. Most MDRAb strains harbor an antibiotic resistance island (AbaR) in their genome, which provides great potential for antibiotic resistance. Moreover, in the present study, we observed that these gene cassettes are shared among these strains and other species from the integron database [29], which provides a clue for the transmission of integrons among inner- and interspecies. Interestingly, several environmental isolates in our ICU possessed a class 1 integrase from a previous collection, while 1 isolate was detected with an empty gene cassette (data not shown). Similarly, there is a recent report that shows 1 environmental isolate of P. aeruginosa with an empty cassette [35]. This empty cassette potentially represents a reservoir with an increased capacity to adapt to the environment.
In conclusion, we found that there are 2 predominant distinct clones (ST92 and ST75) of CNSAb isolates, which clustered to CC92 in our medical ICU. ST92-A. baumannii is considered to be the clone with a worldwide dissemination, while ST75 could be another major clone accompanying the CNSAb isolates in our region. Moreover, the ST75 clone might act as a severe epidemic marker in the carbapenem-resistant A. baumannii isolates in our area. Therefore, CC92-CNSAb plays an important role in causing nosocomial infections in our medical ICU and thus, should be investigated as part of hospital infection control in the future.
Figures and Tables
Fig. 1
Population structure of Acinetobacter baumannii in this study and an existing isolate in China, represented by an eBURST algorithm. A circle represents an ST, and its size corresponds to the number of isolates. The broken line indicates clonal complex (CC) 92. ST112, ST145, ST345, and STn1 are the singletons in this study. ST represents sequence type, i.e. ST92, 1-3-3-2-2-7-3, ST75, 1-3-3-2-2-11-3, ST112, 1-12-56-36-4-61-26, ST145, 21-35-2-28-1-52-4, ST345, 46-12-110-1-16-141-50, and STn1, representing a new sequence type with a G-A mutation at nt268 on the rpoD40 loci of ST251(32-48-54-35-1-11-40); CC represents clonal complex.

Table 2
Distribution, allelic profiles, MIC ranges of IPM and MEM and antibiotic resistance determinants of the isolates
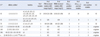
*ST represents sequence type, and the novel unassigned STs were designated STn; †Allelic profiles represent 7 loci in the order of gltA, gyrB, gdhB, recA, cpn60, gpi, and rpoD.
Abbreviations: IPM, imipenem; MEM, meropenem; int1, class 1 integrase; CC, clonal complex (assigned based on eBURST analysis).
Acknowledgement
The authors thank Peinan Wu and other colleagues for recovering and identifying the A. baumannii isolates. This study was supported by the grant from the Nature & Science project in Suzhou (SYSD2010136).
References
1. Joly-Guillou ML. Clinical impact and pathogenicity of Acinetobacter. Clin Microbiol Infect. 2005. 11:868–873.

2. Abbo A, Navon-Venezia S, Hammer-Muntz O, Krichali T, Siegman-Igra Y, Carmeli Y. Multidrug-resistant Acinetobacter baumannii. Emerg Infect Dis. 2005. 11:22–29.
3. Piazza O, Iasiello A, PapaIanni C, De Robertis E, Servillo G, Rossano F, et al. Incidence of antimicrobial-resistant ventilator associated pneumonia: an eighteen-month survey. Panminerva Med. 2005. 47:265–267.
4. Wu CJ, Lee HC, Lee NY, Shih HI, Ko NY, Wang LR, et al. Predominance of Gram-negative bacilli and increasing antimicrobial resistance in nosocomial bloodstream infections at a university hospital in southern Taiwan, 1996-2003. J Microbiol Immunol Infect. 2006. 39:135–143.
5. Jeon BC, Jeong SH, Bae IK, Kwon SB, Lee K, Young D, et al. Investigation of a nosocomial outbreak of imipenem-resistant Acinetobacter baumannii producing the OXA-23 beta-lactamase in korea. J Clin Microbiol. 2005. 43:2241–2245.
6. Lee K, Yum JH, Yong D, Lee HM, Kim HD, Docquier JD, et al. Novel acquired metallo-beta-lactamase gene, bla(SIM-1), in a class 1 integron from Acinetobacter baumannii clinical isolates from Korea. Antimicrob Agents Chemother. 2005. 49:4485–4491.
7. Poirel L, Marque S, Heritier C, Segonds C, Chabanon G, Nordmann P. OXA-58, a novel class D {beta}-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agents Chemother. 2005. 49:202–208.
8. Turton JF, Ward ME, Woodford N, Kaufmann ME, Pike R, Livermore DM, et al. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol Lett. 2006. 258:72–77.

9. Brown S, Young HK, Amyes SG. Characterisation of OXA-51, a novel class D carbapenemase found in genetically unrelated clinical strains of Acinetobacter baumannii from Argentina. Clin Microbiol Infect. 2005. 11:15–23.

10. Merkier AK, Centron D. Bla(OXA-51)-type beta-lactamase genes are ubiquitous and vary within a strain in Acinetobacter baumannii. Int J Antimicrob Agents. 2006. 28:110–113.
11. Zhou H, Pi BR, Yang Q, Yu YS, Chen YG, Li LJ, et al. Dissemination of imipenem-resistant Acinetobacter baumannii strains carrying the ISAba1 blaOXA-23 genes in a Chinese hospital. J Med Microbiol. 2007. 56:1076–1080.
12. White PA, McIver CJ, Rawlinson WD. Integrons and gene cassettes in the enterobacteriaceae. Antimicrob Agents Chemother. 2001. 45:2658–2661.
13. Gu B, Tong M, Zhao W, Liu G, Ning M, Pan S. Prevalence and characterization of class I integrons among Pseudomonas aeruginosa and Acinetobacter baumannii isolates from patients in Nanjing, China. J Clin Microbiol. 2007. 45:241–243.
14. Fu Y, Zhou J, Zhou H, Yang Q, Wei Z, Yu Y, et al. Wide dissemination of OXA-23-producing carbapenem-resistant Acinetobacter baumannii clonal complex 22 in multiple cities of China. J Antimicrob Chemother. 2010. 65:644–650.

15. He C, Xie Y, Zhang L, Kang M, Tao C, Chen Z, et al. Increasing imipenem resistance and dissemination of the ISAba1-associated blaOXA-23 gene among Acinetobacter baumannii isolates in an intensive care unit. J Med Microbiol. 2011. 60:337–341.
16. Adams-Haduch JM, Onuoha EO, Bogdanovich T, Tian GB, Marschall J, Urban CM, et al. Molecular epidemiology of carbapenem-nonsusceptible Acinetobacter baumannii in the United States. J Clin Microbiol. 2011. 49:3849–3854.

17. Chang HC, Wei YF, Dijkshoorn L, Vaneechoutte M, Tang CT, Chang TC. Species-level identification of isolates of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex by sequence analysis of the 16S-23S rRNA gene spacer region. J Clin Microbiol. 2005. 43:1632–1639.
18. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals, 3rd ed., approved standard M31-A3. 2008. Wayne, PA: CLSI, Clinical and Laboratory Standards Institute.
19. Lee K, Lim YS, Yong D, Yum JH, Chong Y. Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-beta-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2003. 41:4623–4629.
20. Bartual SG, Seifert H, Hippler C, Luzon MA, Wisplinghoff H, Rodriguez-Valera F. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J Clin Microbiol. 2005. 43:4382–4390.
21. Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol. 2004. 186:1518–1530.

22. Wang F, Zhu D, Hu F, Ruan F, Ni Y, Sun J, et al. CHINET 2007 Surveillance of bacterial resistance in China (Chinese). Zhongguo Gan Ran Yu Hua Liao Za Zhi. 2008. 8:325–334.
23. Zhang L, Yang W, Xiao M, Xu Y, Zheng B, Lü Y. Mohnarin annual report 2010:surveillance of antimicrobial resistance in bacteria isolated from intensive care units (Chinese). Zhonghua Yi Yuan Gan Ran Xue Za Zhi. 2012. 28:330–335.
24. Zhang JP, Zhu W, Chu YZ, Tian SF, Chen BY. Molecular epidemiological study of multi-drug resistant Acinetobacter baumannii (Chinese). Zhonghua Nei Ke Za Zhi. 2010. 49:657–661.
25. Grosso F, Carvalho KR, Quinteira S, Ramos A, Carvalho-Assef AP, Asensi MD, et al. OXA-23-producing Acinetobacter baumannii: a new hotspot of diversity in Rio de Janeiro? J Antimicrob Chemother. 2011. 66:62–65.

26. Wang H, Guo P, Sun H, Yang Q, Chen M, Xu Y, et al. Molecular epidemiology of clinical isolates of carbapenem-resistant Acinetobacter spp. from Chinese hospitals. Antimicrob Agents Chemother. 2007. 51:4022–4028.
27. Lei J, Yuan L, Li H, Zeng X. Study of metallo-β-lactamase of Carbapenems-resistant Acinetobacter baumanii (Chinese). Xian Dai Zhong Xi Yi Jie He Za Zhi. 2009. 27:3280–3281.
28. Dawes FE, Kuzevski A, Bettelheim KA, Hornitzky MA, Djordjevic SP, Walker MJ. Distribution of class 1 integrons with IS26-mediated deletions in their 3'-conserved segments in Escherichia coli of human and animal origin. PLoS One. 2010. 5:e12754.

29. Moura A, Soares M, Pereira C, Leitao N, Henriques I, Correia A. INTEGRALL: a database and search engine for integrons, integrases and gene cassettes. Bioinformatics. 2009. 25:1096–1098.

30. Xu X, Kong F, Cheng X, Yan B, Du X, Gai J, et al. Integron gene cassettes in Acinetobacter spp. strains from South China. Int J Antimicrob Agents. 2008. 32:441–445.

31. Hornsey M, Loman N, Wareham DW, Ellington MJ, Pallen MJ, Turton JF, et al. Whole-genome comparison of two Acinetobacter baumannii isolates from a single patient, where resistance developed during tigecycline therapy. J Antimicrob Chemother. 2011. 66:1499–1503.

32. Fournier PE, Vallenet D, Barbe V, Audic S, Ogata H, Poirel L, et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006. 2:e7.

33. Zhou H, Zhang T, Yu D, Pi B, Yang Q, Zhou J, et al. Genomic analysis of the multidrug-resistant Acinetobacter baumannii strain MDR-ZJ06 widely spread in China. Antimicrob Agents Chemother. 2011. 55:4506–4512.

34. Krizova L, Dijkshoorn L, Nemec A. Diversity and evolution of AbaR genomic resistance islands in Acinetobacter baumannii strains of European clone I. Antimicrob Agents Chemother. 2011. 55:3201–3206.

35. Ruiz-Martinez L, Lopez-Jimenez L, Fuste E, Vinuesa T, Martinez JP, Vinas M. Class 1 integrons in environmental and clinical isolates of Pseudomonas aeruginosa. Int J Antimicrob Agents. 2011. 38:398–402.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download