Abstract
Background
Stenotrophomonas maltophilia has emerged as an important opportunistic pathogen, which causes infections that are often difficult to manage because of the inherent resistance of the pathogen to a variety of antimicrobial agents. In this study, we analyzed the expressions of smeABC and smeDEF and their correlation with antimicrobial susceptibility. We also evaluated the genetic relatedness and epidemiological links among 33 isolates of S. maltophilia.
Methods
In total, 33 S. maltophilia strains were isolated from patients in a tertiary hospital in Daejeon. Minimum inhibitory concentrations (MICs) of 11 antimicrobial agents were determined by using agar dilution method and E-test (BioMérieux, France). Real-time PCR analysis was performed to evaluate the expression of the Sme efflux systems in the S. maltophilia isolates. Additionally, an epidemiological investigation was performed using multilocus sequence typing (MLST) assays.
Results
The findings of susceptibility testing showed that the majority of the S. maltophilia isolates were resistant to β-lactams and aminoglycosides. Twenty-one clinical isolates overexpressed smeABC and showed high resistance to ciprofloxacin. Moreover, a high degree of genetic diversity was observed among the S. maltophilia isolates; 3 sequence types (STs) and 23 allelic profiles were observed.
Conclusions
The smeABC efflux pump was associated with multidrug resistance in clinical isolates of S. maltophilia. In particular, smeABC efflux pumps appear to perform an important role in ciprofloxacin resistance of S. maltophilia. The MLST scheme for S. maltophilia represents a discriminatory typing method with stable markers and is appropriate for studying population structures.
Stenotrophomonas maltophilia is a non-fermentative gram-negative bacillus found extensively in the environment and has emerged as an important nosocomial pathogen [1, 2]. S. maltophilia causes a variety of nosocomial infections, particularly pneumonia and bacteremia in severely debilitated or immunosuppressed patients with underlying chronic diseases who are admitted to intensive care units. Its infamy is partly attributable to its characteristic resistance to most of the currently available broad-spectrum antimicrobial agents and partly to its ability to rapidly proliferate a multiresistant phenotype [3-5].
S. maltophilia has high intrinsic resistance to a variety of structurally unrelated antimicrobial agents, including β-lactams, aminoglycosides, and quinolones. In part, the resistance of S. maltophilia to multiple antimicrobial agents can be attributed to limited outer membrane permeability and active antimicrobial efflux; 2 Sme efflux systems, smeABC and smeDEF, have been identified in this organism [6-8]. The mechanisms underlying antimicrobial drug resistance in clinical isolates have proven problematic in the treatment of S. maltophilia infections. The recently developed multilocus sequence typing (MLST) technique appears to be a reliable tool for tracking the source of infection and the distribution of pathogens isolated from hospitalized patients; this technique provides consistent epidemiological data, and the results from different laboratories can be compared because the international databases are easily accessible [9-11].
The objectives of the present study were to correlate the antimicrobial resistance patterns of the S. maltophilia isolates with their antimicrobial efflux mechanisms and to determine the genetic characteristics of S. maltophilia isolates that are responsible for their current epidemic status.
In total, 33 consecutive, non-duplicated S. maltophilia isolates were obtained from patients in a tertiary hospital in Daejeon, Korea between January and December 2009. Biochemical profiling using conventional methods and the Vitek 2 system (BioMérieux, Hazelwood, MO, USA) for microbial identification were used to confirm that the isolates were S. maltophilia.
In the antimicrobial susceptibility tests, minimum inhibitory concentration (MIC) was determined by using the agar dilution method and an E-test conducted in accordance with the guidelines of the CLSI [12, 13]. The susceptibility of S. maltophilia to the following antimicrobial agents was tested: ceftazidime (Hanmi, Seoul, Korea), cefepime (Boryung, Seoul, Korea), ticarcillin/clavulanic acid, meropenem, aztreonam, trimethoprim/sulfamethoxazole (bioMérieux, Marcy I'Etoile, France), amikacin, gentamicin, levofloxacin, minocycline (Sigma Chemical Co., St Louis, MO, USA), and ciprofloxacin (Fluka, Buchs, Switzerland). Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used for quality control purposes.
Cell suspensions were prepared and inoculated in brain-heart infusion broth (Difco, Cockeysville, MD, USA). After overnight culture, total RNA was extracted from the cell suspensions by using the TRI REAGENT® (Montgomery, OH, USA). Additionally, 20 µL of cDNA was obtained from 5 µg of total RNA by using the DiaStar™ RT Kit (SolGent, Daejeon, Korea). The amplification mixtures for real-time PCR (20 µL) contained template cDNA, 2× SYBR Green I Master Mix (Applied Biosystems, Foster, CA, USA), and primers.
The sequences of the primers designed for this study were as follows: smeB (F: 5'-ACCGCCCAGCTTTCATACAG-3'; R: 5'-GACATGGCCTACCAGGAACAG-3') and smeF (F: 5'-TCGTCCAGGCTGACATTCAA-3'; R: 5'-AACGCGGATCGTGATATCG-3'). The primer sequences used for the endogenous control gene were rDNA (F: 5'-TGACACTGAGGCACGAAAGC-3'; R: 5'-CATCGTTTAGGGCGTGGACTA-3'). Real-time PCR reactions were performed using the StepOne real-time PCR (Applied Biosystems); the PCR cycle included the following steps: activation and denaturation step for 10 min at 95℃, 40 cycles of 15 sec at 95℃, and annealing and extension for 1 min at 60℃. Standard curves for the expression levels of smeABC, smeDEF, and rDNA were constructed using the expression levels of these genes in S. maltophilia ATCC 13637; these standard curves were used as calibrators to normalize the relative expression levels of the smeB and smeF genes in clinical isolates. Overexpression of the Sme efflux system was assessed as described in the study of Chang et al. [7].
Genomic DNA was extracted from the clinical isolates by using a Genomic DNA Prep Kit (SolGent) and was used as a template. PCR was performed using 50 ng of the template DNA (genomic DNA), 2.5 µL of 10×Taq buffer, 0.5 µL of 10 mM dNTP mix, 20 pmol of each primer, and 0.7 U of Taq DNA Polymerase (SolGent) in a total volume of 25 µL. Internal fragments of 7 housekeeping genes (atpD, gapA, guaA, mutM, nuoD, ppsA, and recA) were amplified in a GeneAmp PCR System 9600 (Perkin-Elmer Cetus Corp., Norwalk, CT, USA) by using the following steps: pre-denaturation of the reaction mixture for 9 min at 95℃; 30 cycles of 20 sec at 94℃, 1 min at appropriate annealing temperature, and 50 sec at 72℃; and final elongation for 5 min at 72℃ [9]. The amplicons were purified using a PCR Purification Kit (SolGent) and sequenced using a BigDye Terminator Cycle Sequencing Kit (Applied Biosystems) and an ABI PRISM 3730XL DNA Analyzer (Applied Biosystems). The allele number for each gene was assigned on the basis of the information in the S. maltophilia MLST database (http://pubmlst.org/smaltophilia/). A combination of the allelic sequences of the 7 genes yielded the allelic profile for each isolate.
We tested the susceptibility of 33 clinical isolates of S. maltophilia to 11 antimicrobial agents on the basis of the MIC determinations (Table 1). S. maltophilia showed low susceptibility to cefepime (9.1%), meropenem (0%), and aztreonam (3.0%), which are members of the β-lactam group; however, it showed relatively high susceptibility to ceftazidime (69.7%). The susceptibility of the organism to aminoglycosides, namely, amikacin and gentamicin, was 6.1%, whereas that for ciprofloxacin (a quinolone) was 45.5%. Most of the isolates were susceptible to ticarcillin/ clavulanic acid (97.0%), levofloxacin (87.9%), trimethoprim/sulfamethoxazole (93.9%), and minocycline (97.0%).
Real-time PCR analysis was used to assess the overexpression of the Sme efflux systems (smeABC and smeDEF), and the results of the analysis showed overexpression of smeB in 21 (63.6%) and smeF in 19 (57.5%) of the 33 clinical isolates. Fifteen (45.4%) isolates overexpressed both smeB and smeF (Table 2). After analyzing the pattern of antimicrobial resistance and the overexpression of Sme efflux systems, the MICs of ciprofloxacin (MIC50=4; 6.83-fold increase) and levofloxacin (MIC50=1; 3.60-fold increase) were found to be significantly higher for the isolates with detectable smeB and/or smeF than for the isolates without detectable smeB and/or smeF. Overexpression of the smeABC efflux pump was statistically related to the MICs of ciprofloxacin (P=0.033) and levofloxacin (P=0.034). However, the overexpression of smeDEF (CIP, P=0.100; LEV, P=0.162) alone and both smeABC and smeDEF (CIP, P=0.056; LEV, P=0.064) were not related to quinolone resistance.
In the MLST analysis of the 33 S. maltophilia isolates, 3 sequence types (STs) (ST5, ST28, and ST31) and 23 diverse allelic profiles were detected (Table 1). The most common form of allelic profile was 19, 5, 14, 8, 5, 26, 4 (4 isolates); the other forms were ST31 (3, 4, 24, 7, 7, 22, 7; 2 isolates), ST5 (5, 22, 9, 4, 27, 5, 7; 2 isolates), 8, 20, 10, 25, 14, 17, 3 (2 isolates), and 2, 13, 42, 20, 30, 27, 20 (2 isolates).
The NJ tree model was chosen for representing the findings of the cluster analysis of all 33 S. maltophilia strains (Fig. 1).
S. maltophilia was first reported in the early 1970s, and since then, it has continued to spread through the 1990s. This spread can be attributed to the widespread use of chemotherapy and broad-spectrum antimicrobial agents as well as to the adoption of highly invasive medical practices [14]. Among the clinically important bacteria isolated from major hospitals in Korea, S. maltophilia had isolation rates of 1.8% in 1998 and 3.1% in 2005 and 2006; these isolation rates are indicative of a serious problem of nosocomial infection [15, 16].
S. maltophilia is intrinsically resistant to multiple antimicrobial agents, including β-lactams, aminoglycosides, carbapenems, and quinolones; clinical isolates of S. maltophilia frequently exhibit high multidrug resistance. In this study, 33 S. maltophilia isolates were found to be highly resistant to β-lactams and aminoglycosides; these organisms showed a ciprofloxacin susceptibility rate of 45.5%. According to the findings of a survey conducted in Korea between 2000 and 2003, the rate of ciprofloxacin resistance was approximately 10% [17]. However, in this study, the organism showed a significant 3-fold increase in drug resistance.
Although various mechanisms for antimicrobial drug resistance have been reported in clinical isolates of S. maltophilia thus far, little information is available about this species in Korea. Recently, antimicrobial efflux mechanisms have been increasingly recognized as major factors in the intrinsic and acquired resistance of many significant human pathogens, including Pseudomonas aeruginosa and Burkholderia cepacia. In particular, antimicrobial efflux systems (including MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY-OprM) belonging to the resistance-nodulation-division family, which contribute to multidrug resistance have been characterized in P. aeruginosa [18-20]. Additionally, overexpression of the smeABC and SmeDEF efflux pumps in S. maltophilia contributes to intrinsic multidrug resistance and plays an important role in the resistance characteristics of clinical isolates. There have been reports that the overexpression of the Sme efflux system of S. maltophilia induced resistance to several antimicrobial agents, including β-lactams, aminoglycosides, and quinolones [6, 7, 21, 22]. We found that the overexpression rates of smeB and smeF were 63.6% and 57.4%, respectively, in organisms with high resistance to β-lactams and aminoglycosides. A study conducted in Taiwan in 2004 indicated that the smeABC efflux pumps might play a role in the ciprofloxacin resistance of S. maltophilia [7]. In this study, 21 isolates with smeB, which was detected using real-time PCR analysis, showed a 6.83-fold and 3.60-fold increase in the MICs of ciprofloxacin and levofloxacin, respectively. The MIC of ciprofloxacin in our study was 3-fold higher than that in a previous Taiwanese report (2.16-fold). Thus, we suggest that the overexpression of smeABC was associated with high MIC values for ciprofloxacin (P=0.033) and levofloxacin (P=0.034). However, the overexpression of SmeDEF (CIP, P=0.100; LEV, P=0.162) alone and both smeABC and SmeDEF (CIP, P=0.056; LEV, P=0.064) were not related to quinolone resistance.
MLST for molecular epidemiologic studies of S. maltophilia is a recently developed strain-typing system that focuses strictly on conserved housekeeping genes. In previous studies in Korea, this genetic relationship was evaluated using pulsed-filled gel electrophoresis (PFGE) after XbaI digestion or molecular typing by using enterobacterial repetitive intergenic consensus (ERIC)-PCR and random amplified polymorphic DNA (RAPD) [17, 23]. It is thought that phylogenetic studies of S. maltophilia by using MLST will contribute significantly to a new perspective on the molecular epidemiology of S. maltophilia. We applied MLST analysis to 33 strains and found that most of the strains represent 3 STs (ST5, ST28, and ST31) as well as 23 new allelic profiles. Similarly, studies in Spain in 2004 and in Korea in 2010 showed a high degree of genetic diversity among S. maltophilia isolates despite their origin in a single hospital [5, 23]. This finding indicates that S. maltophilia has a high potential for environmental distribution. In the S. maltophilia MLST database (http://www.pubmlst.org/smaltophilia/), 56 STs have been reported thus far, of which ST5, ST28, and ST31 have been identified in Korea. Database analysis shows that there are considerably fewer STs for S. maltophilia isolates than other bacterial isolates.
A distinct finding of our study was the relationship between quinolone resistance and the overexpression of the smeABC efflux system. This relationship was also observed in some parts of the MLST cluster. The group A and A' isolates shown in Fig. 1 were highly resistant to ciprofloxacin and harbored smeB, as observed in the real-time PCR analysis. Additionally, the group B isolates were highly resistant to ceftazidime and cefepime (Fig. 1). A similar relationship exists between the clonal complex 17 (CC17) and antimicrobial resistance of Enterococcus faecium. CC17 was defined by using an MLST assay and is regarded as excellent example of cumulative evolutionary processes. In the majority of the E. faecium isolates, CC17 is characterized by ampicillin and quinolone resistance as well as the presence of a putative pathogenicity island, including the esp gene [24, 25].
The incidence of S. maltophilia infections has increased worldwide, but only a few studies on this topic have been performed in Korea thus far. On the basis of the findings of the MLST assays for S. maltophilia, we found that antimicrobial resistance correlated with the overexpression of the Sme efflux systems.
Figures and Tables
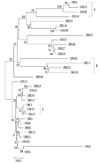 | Fig. 1A phylogenetic tree of the concatenated nucleotide sequences of 7 housekeeping genes (atpD, gapA, guaA, mutM, nuoD, ppsA, and recA) of S. maltophilia obtained using the NJ method with Kimura 2 correction for distance calculations. Group A and A' isolates were highly resistant to ciprofloxacin and overexpressed smeB, and Group B isolates were highly resistant to ceftazidime and cefepime. |
Table 1
MLST, antimicrobial susceptibility, and mRNA expression studies for the 33 Stenotrophomonas maltophilia isolates

Abbreviations: MLST, multilocus sequence typing; MIC, minimum inhibitory concentration; CAZ, ceftazidime; FEP, cefepime; TIM, ticarcillin/clavulanic acid; MEM, meropenem; ATM, aztreonam; AMK, amikacin; GEN, gentamicin; CIP, ciprofloxacin; LEV, levofloxacin; SXT, trimethoprim/sulfamethoxazole; MIN, minocycline.
References
1. Hu LF, Chang X, Ye Y, Wang ZX, Shao YB, Shi W, et al. Stenotrophomonas maltophilia resistance to trimethoprim/sulfamethoxazole mediated by acquisition of sul and dfrA genes in a plasmid-mediated class 1 integron. Int J Antimicrob Agents. 2011. 37:230–234.
2. Liaw SJ, Lee YL, Hsueh PR. Multidrug resistance in clinical isolates of Stenotrophomonas maltophilia: roles of integrons, efflux pumps, phosphoglucomutase (SpgM), and melanin and biofilm formation. Int J Antimicrob Agents. 2010. 35:126–130.
3. San Gabriel P, Zhou J, Tabibi S, Chen Y, Trauzzi M, Saiman L. Antimicrobial susceptibility and synergy studies of Stenotrophomonas maltophilia isolates from patients with cystic fibrosis. Antimicrob Agents Chemother. 2004. 48:168–171.
4. Falagas ME, Valkimadi PE, Huang YT, Matthaiou DK, Hsueh PR. Therapeutic options for Stenotrophomonas maltophilia infections beyond cotrimoxazole: a systematic review. J Antimicrob Chemother. 2008. 62:889–894.
5. Valdezate S, Vindel A, Martín-Dávila P, Del Saz BS, Baquero F, Cantón R. High genetic diversity among Stenotrophomonas maltophilia strains despite their originating at a single hospital. J Clin Microbiol. 2004. 42:693–699.
6. Li XZ, Zhang L, Poole K. SmeC, an outer membrane multidrug efflux protein of Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 2002. 46:333–343.
7. Chang LL, Chen HF, Chang CY, Lee TM, Wu WJ. Contribution of integrons, and smeABC and SmeDEF efflux pumps to multidrug resistance in clinical isolates of Stenotrophomonas maltophilia. J Antimicrob Chemother. 2004. 53:518–521.
8. Nicodemo AC, Araujo MR, Ruiz AS, Gales AC. In vitro susceptibility of Stenotrophomonas maltophilia isolates: comparison of disc diffusion, Etest and agar dilution methods. J Antimicrob Chemother. 2004. 53:604–608.
9. Kaiser S, Biehler K, Jonas D. A Stenotrophomonas maltophilia multilocus sequence typing scheme for inferring population structure. J Bacteriol. 2009. 191:2934–2943.
10. Foley SL, Lynne AM, Nayak R. Molecular typing methodologies for microbial source tracking and epidemiological investigations of gram-negative bacterial foodborne pathogens. Infect Genet Evol. 2009. 9:430–440.

11. Schaumann R, Laurin F, Rodloff AC. Molecular typing of clinical isolates of Stenotrophomonas maltophilia by pulsed-field gel electrophoresis and random primer PCR fingerprinting. Int J Hyg Environ Health. 2008. 211:292–298.
12. National Committee for Clinical Laboratory Standards. Approved standard NCCLS document M7-A6. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. 2003. 6th ed. Wayne, PA: CLSI.
13. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twentieth informational supplement M100-S20. 2010. Wayne, PA: Clinical and Laboratory Standards Institute.
14. Denton M, Kerr KG. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin Microbiol Rev. 1998. 11:57–80.
15. Lee K, Chang CL, Lee NY, Kim HS, Hong KS, Cho HC. Korean nationwide surveillance of antimicrobial resistance of bacteria in 1998. Yonsei Med J. 2000. 41:497–506.

16. Lee H, Kim CK, Lee J, Lee SH, Ahn JY, Hong SG, et al. Antimicrobial resistance of clinically important bacteria isolated from 12 hospitals in Korea in 2005 and 2006. Korean J Clin Microbiol. 2007. 10:59–69.
17. Jang KS, Oh JY, Kang HY, Jin JS, Seol SY, Kim J, et al. Genetic diversity of Stenotrophomonas maltophilia isolated from clinical specimens. J Bacteriol Virol. 2007. 37:79–89.
18. Poole K. Efflux-mediated multiresistance in gram-negative bacteria. Clin Microbiol Infect. 2004. 10:12–26.

19. Quale J, Bratu S, Gupta J, Landman D. Interplay of efflux system, ampC, and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother. 2006. 50:1633–1641.
20. Zhang L, Li XZ, Poole K. Multiple antibiotic resistance in Stenotrophomonas maltophilia: involvement of a multidrug efflux system. Antimicrob Agents Chemother. 2000. 44:287–293.
21. Alonso A, Morales G, Escalante R, Campanario E, Sastre L, Martinez JL. Overexpression of the multidrug efflux pump SmeDEF impairs Stenotrophomonas maltophilia physiology. J Antimicrob Chemother. 2004. 53:432–434.
22. Gould VC, Avison MB. SmeDEF-mediated antimicrobial drug resistance in Stenotrophomonas maltophilia clinical isolates having defined phylogenetic relationships. J Antimicrob Chemother. 2006. 57:1070–1076.
23. Song JH, Sung JY, Kwon KC, Park JW, Cho HH, Shin SY, et al. Analysis of acquired resistance genes in Stenotrophomonas maltophilia. Korean J Lab Med. 2010. 30:295–300.
24. Top J, Willems R, van der Velden S, Asbroek M, Bonten M. Emergence of clonal complex 17 Enterococcus faecium in the Netherlands. J Clin Microbiol. 2008. 46:214–219.
25. Cho HH, Sung JY, Kwon KC, Lim JS, Koo SH. Antimicrobial resistance and multilocus sequence typing of vancomycin-resistant Enterococcus faecium isolated from the Chungcheong area. Korean J Clin Microbiol. 2011. 14:60–66.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download