Abstract
Background
Major burn injury induces an inflammatory response that is accompanied by the release of various cytokines. We investigated the gradual changes in the levels of pro-inflammatory and anti-inflammatory cytokines following burn injury and determined the relationship between these levels and burn size in adult Korean patients with burn injury.
Methods
Blood samples from 9 healthy controls and 60 Korean burn patients were collected on days 1, 3, 7, 14, and 21 after burn injury, and concentrations of interleukin (IL)-6, IL-8, IL-10, tumor necrosis factor (TNF)-α, and granulocyte-colony stimulating factor (G-CSF) were measured. Burn patients were divided into 3 groups according to burn size (15-30%, 31-50%, >50% total body surface area), and the concentrations of the cytokines were compared between these groups and the control group over 3 weeks.
Results
Compared to their levels in controls, IL-6, IL-8, IL-10, TNF-α, and G-CSF levels in burn patients were significantly higher during the observation period. Median concentrations of IL-8, IL-10, and G-CSF at each time point increased with burn size, although peak levels and time to peak levels of these cytokines differed from patient to patient.
Major burn injury induces an inflammatory response, which is accompanied by the release of various cytokines [1]. During this response, pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor (TNF)-α, or interferon (IFN)-γ, and anti-inflammatory cytokines such as IL-4, IL-10, and granulocyte-colony stimulating factor (G-CSF) are released. Inflammation is controlled by the balance between these pro- and anti-inflammatory mediators; simultaneous production of anti-inflammatory cytokines may counteract the effects of pro-inflammatory cytokines and modify the intensity of the inflammatory response. Uncontrolled release of pro- and anti-inflammatory cytokines promotes immunological dysfunction that results in significant morbidity [1]. If systemic immunity fails to restore the integrity of the host, the immune system dysregulation can result in significant systemic inflammation and immune paralysis; these uncontrolled systemic immune events can eventually lead to tissue damage and multiple organ failure.
Several studies have reported that the levels of various cytokines are high in burn patients; increased cytokine levels are also related to sepsis and mortality in these patients [1-6]. As increased burn size also leads to increased mortality in burn patients, it is plausible that increased burn size is related to increased cytokine concentrations; however, this may not be true for all cytokines. One study in a children's hospital in Texas, USA, reported that the levels of IL-8, TNF, IL-6, IL-12p70, monocyte chemotactic protein, and granulocyte macrophage-colony stimulating factor were significantly high in large burns, but that those of other cytokines, including IL-1β, IFN-γ, IL-2, IL-4, and IL-10, were not affected by burn size in pediatric burn patients [4]. In another study, IL-6 levels were correlated with burn size, but TNF-α and IL-1β levels were not [7]; similarly, Kowal-Vern et al. found that IL-6 levels increased in proportion to burn size, but IL-2 levels did not [8]. In yet another study, IL-1β concentration was positively correlated with burn size, but IL-6 and TNF-α levels were not [9].
Some cytokine gene polymorphisms occur with different allele frequencies in different ethnic groups [10-12]; similarly, cytokine levels also differ between different groups. For instance, serum IL-6 concentration was different between Japanese and American individuals [13], and placental cytokine responses, namely IL-1, IL-6, IL-8, and IL-10, to preterm birth also differed between African-American and Caucasian individuals [14]. Therefore, cytokine responses to burn injury could also differ amongst different ethnic groups; however, to date, various cytokine concentrations have not been studied in Korean adult burn patients.
Therefore, the aim of this study was to investigate the levels of pro-inflammatory cytokines (IL-6, IL-8, and TNF-α) and anti-inflammatory cytokines (IL-10 and G-CSF) over time, and to determine the relationship between the levels of these cytokines and burn size in an adult Korean burn patient population.
Sixty burn patients participated in this prospective study. Patients met the following criteria: individuals 18-70 yr of age, having arrived at a participating hospital within 24 hr after the injury, and having burns covering over 15% of the total burn surface area (TBSA). Patients' clinical data, including information on the age, sex, type of burn, TBSA, admission period, hospital course, and results of white blood cell (WBC) count, C-reactive protein (CRP) measurement, blood culture, and wound culture during the admission period were collected. For controls, sera from 9 healthy adults were collected (age, 28-46 yr). The study protocols were approved by the Institutional Review Board of the Hangang Sacred Heart Hospital, and informed consent was obtained from each patient or a family representative.
Blood samples were collected on days 1, 3, 7, 14, and 21 after burn injury. Blood was collected into siliconized vacutainer tubes containing EDTA as anticoagulant and was processed within 30 min of collection. The tubes were centrifuged at 3,000 rpm (2,092 g) for 10 min, after which plasma was removed and stored in aliquots at -70℃ until analysis. IL-6, IL-8, IL-10, TNF-α, and G-CSF levels were measured using a bead array assay kit from Millipore Corp. (St. Charles, MO, USA) with the Bio-Plex Suspension Array System (Bio-Rad, Hercules, CA, USA), according to the manufacturer's instructions. In brief, plasma samples were thawed, centrifuged at 3,000 rpm for 5 min, and then incubated with microbeads labeled with specific antibodies to the above cytokines for 30 min. After a wash step, the beads were incubated with the detection antibody cocktail, with each antibody specific to a single cytokine. After another wash step, the beads were incubated with streptavidin-phycoerythrin for 10 min and again washed, and the concentrations of each cytokine then determined using an array reader. All of cytokine measurements were conducted duplicated, and were conducted at the same time for healthy controls and burn patients.
Data are expressed as mean±SD (range) or median (interquartile range) values for each group of patients. Missing data at each time point (which could not be recorded due to the patient's death or a missed sampling) were excluded to calculate the median (interquartile range). Statistical differences in cytokine concentrations among control and patient groups at different time points (days 1, 3, 7, 14, and 21) were analyzed using the independent two-sample Mann-Whitney U test and Friedman test. Data from patient groups with different burn sizes were analyzed at each time point by the Kruskal-Wallis test and post-hoc analysis. Results were considered statistically significant when the P values <0.05, and Bonferroni correction was used to adjust P values (corrected P) for multiple tests [15]. The MedCalc program (MedCalc version 12.2.1.0, Mariakerke, Belgium) was used for statistical analysis.
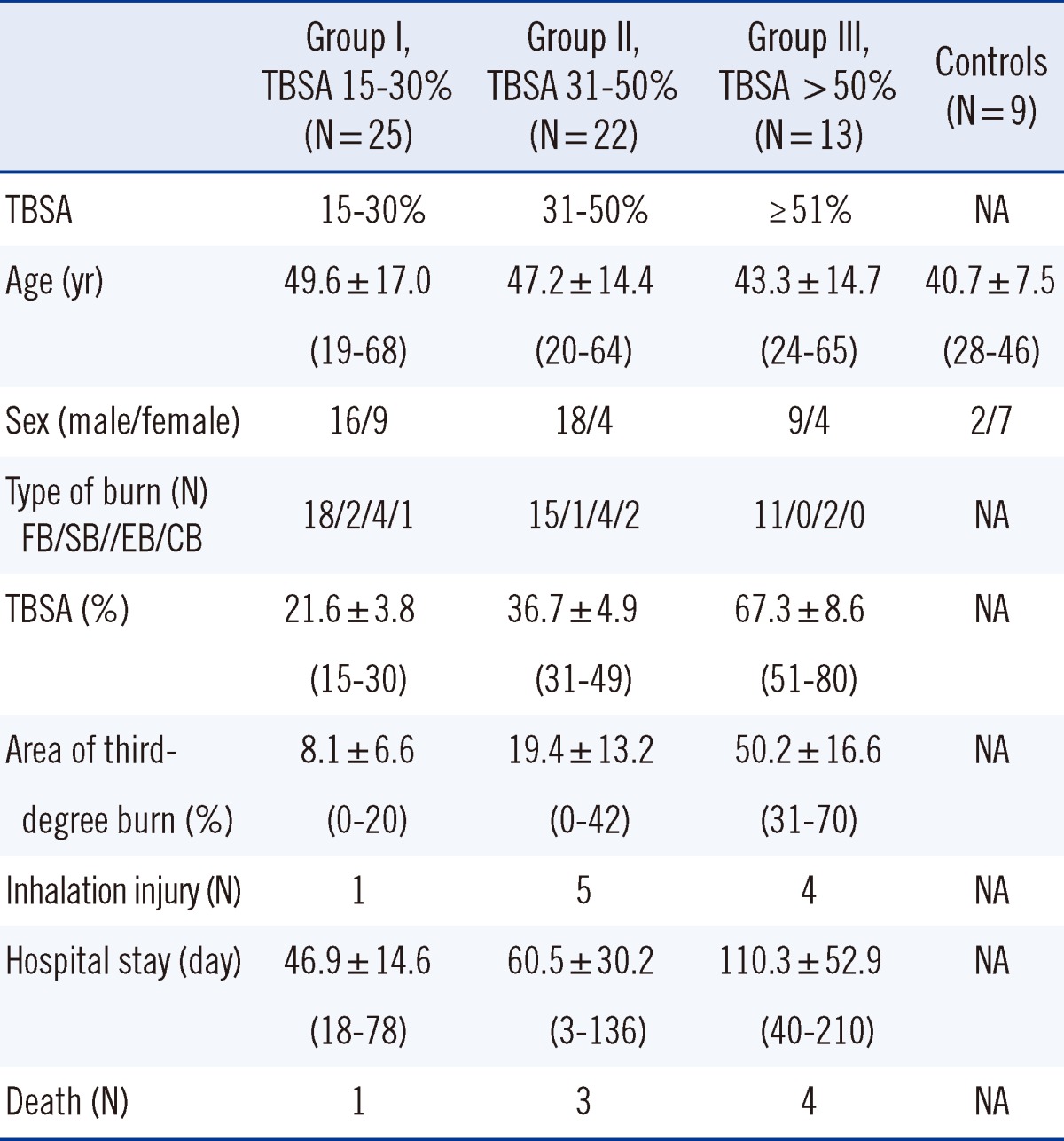
The TBSA of patients ranged from 15% to 80% (mean, 35.7%). Ten patients had additional inhalation injury and 8 of the patients died. The 60 burn patients were classified into 3 groups by burn size; TBSA in Group I was 15-30% (N=24), in Group II, 31-50% (N=19), and in Group III, >50% (N=11); demographic data and patient characteristics are shown in Table 1. There was no statistically significant difference in age among the 3 groups.
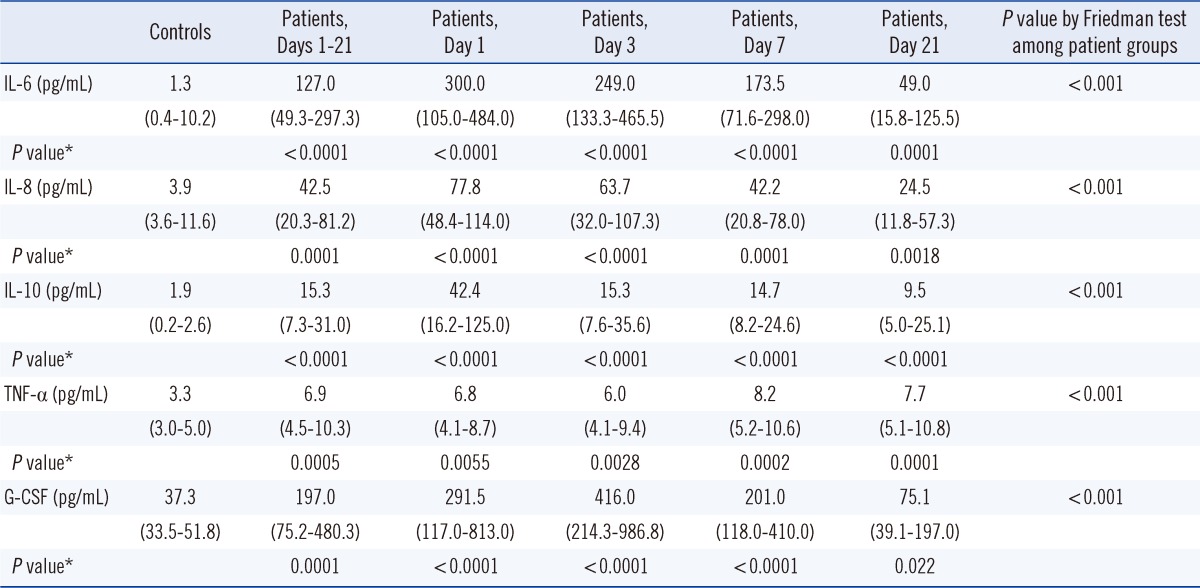
Compared to their levels in non-burn controls, IL-6, IL-8, IL-10, TNF-α, and G-CSF levels in burn patients were significantly higher during the observed period after burn injury (P<0.05) (Table 2). Median (interquartile range) concentrations of IL-6, IL-8, IL-10, TNF-α, and G-CSF in burn patients during the observation period were 127.0 (49.3-297.3) pg/mL, 42.5 (20.3-81.2) pg/mL, 15.3 (7.3-31.0) pg/mL, 6.9 (4.5-10.3) pg/mL, and 197.0 (75.2-480.3) pg/mL, respectively. These levels were significantly higher than those in non-burn controls: 1.3 (0.4-10.2) pg/mL, 3.9 (3.6-11.6) pg/mL, 1.9 (0.2-2.6) pg/mL, 3.3 (3.0-5.0) pg/mL, and 37.3 (33.5-51.8) pg/mL, respectively.
Although peak levels and time to peak levels of these cytokines differed from patient to patient, IL-6 and IL-10 showed the highest level at day 1 and gradually decreased thereafter. IL-8 showed the highest level at day 1 in Groups I and II, but in Group III, the highest IL-8 level was reached at day 7 (Fig. 1). TNF-α level also did not decrease during the observation period. G-CSF concentration showed a tendency to reach the highest level at day 3. There was no consistent correlation between increases in particular cytokine levels and bacterial infection (positive blood culture or wound culture), increased CRP or WBC, or patient prognosis (death or prolonged hospital stay).
Median concentrations of IL-8, IL-10, and G-CSF at each time point increased according to burn size (Fig. 1). However, there was no clear increase in TNF-α levels with an increase in burn size.
In this study, we show that IL-6, IL-8, IL-10, TNF-α, and G-CSF significantly increased during a 3-week observation period after burn injury in adult Korean burn patients compared to non-burn controls. This finding is consistent with those of other studies [1, 5]. Median concentrations of IL-8, IL-10, and G-CSF also increased in accordance with burn size, but the relationship between TNF-α concentration and burn size was unclear (Fig. 1).
Cytokine expression differs between adults and children [3, 16]; moreover, as previously mentioned, the mean cytokine concentration and immune response may differ amongst different ethnic groups. Our study shows, for the first time, the temporal expression pattern of 5 cytokines after burn injury in Korean adult patients. The concentrations of IL-6, IL-8, IL-10, TNF-α, and G-CSF were higher in the burn patients than in non-burn controls, which is consistent with the findings of other studies.
Several studies have analyzed the relationship between burn size and cytokine concentration, although their results varied. Most studies reported that IL-6 and IL-8 concentrations were related to burn size [4, 7-9, 17]. Some reported that IL-10 concentration was related to burn size [18], but others did not find this association [4, 17]. TNF-α level was related to burn size in one study [4], but not in other studies [7, 9, 19]. One explanation for these discrepant results may be that, although an increase in cytokine concentration is correlated overall with burn size, other factors such as fever, infection, and WBC can cause a rapid increase in the cytokine concentration. In fact, as shown in Fig. 1, although the median concentration of IL-10 increased as the burn size increased, the peak concentration in Group II, in which burn size was smaller than in Group III, was higher than that of Group III on postburn day 1, suggesting that factors other than burn size may influence the cytokine concentrations. In our study, extreme elevations in IL-6, IL-10, and G-CSF levels were seen in some patients; the reasons for this were not clear, although we examined common triggering factors such as fever, bacterial infection, and concurrent increases of WBC, CRP, or other cytokines. It may be that cytokine increase is influenced by many factors, including the cytokine network system.
Furthermore, previous studies reported varying results regarding the changes in cytokine levels according to postburn time. Some studies reported that the IL-6 concentration was highest at 1 day after the burn, and then decreased [9], whereas other studies reported that it was highest after 3-4 days [1, 3, 7, 20, 21]. Similarly, IL-8 concentration has been reported as highest after 1 day, followed by a decrease [17], but also as highest after 3-4 days [1, 20, 21] or after 15-21 days [3]. Some studies reported that the IL-10 concentration was highest at day 1 [3, 16, 21], as we found in this study, whereas others reported that it was highest after 3 days [20], 7 days [22], or even 14 days [18]. Some studies reported that the G-CSF concentration was highest after 2 days [3], and other studies reported that TNF-α levels started increasing after 7 days, or was no different from that of the control group [1, 3]. In this study, the concentrations of IL-6, IL-8, and IL-10 began to increase after 1 day and then decreased gradually; the G-CSF concentration was highest after 3 days, and the TNF-α concentration was only slightly higher (approximately 1.5-2.5 times higher) than that in the non-burn control group during the observation period. It is possible that the median concentrations of the cytokines at days 7, 14, and 21 were underestimated, as data from patients who were dead at these times could not be included in the analysis, and severe burn injury resulting in early death was usually associated with higher concentrations of cytokines.
When considering individual data, rather than the average or median value of each group, however, we observed peak cytokine concentrations on all of the observation days (day 1, 3, 7, 14, and 21), indicating that time to peak cytokine levels varies amongst individual subjects. There was also a large degree of overlap in cytokine concentrations between burn patients and non-burn controls, with IL-6, IL-8, IL-10, TNF-α, and G-CSF levels in some control individuals attaining similar levels to those in burn patients.
The differences in the time to peak cytokine concentration between individuals or studies could result from increases in the concentration of the cytokines due to multiple factors, rather than by a single factor, some of which can increase concentrations by tens of thousands of times. It is reported that cytokine concentration fluctuates in seriously ill patients and that a variety of factors affect circulating cytokine levels [23]. For this reason, it is believed that the resulting peak cytokine concentration and time to peak level cannot be generalized. The different patterns for each cytokine suggest distinct and different roles for each of the cytokines in modulating immunological and inflammatory responses after severe burns.
Thus, it is believed that the increase in cytokine concentrations after a burn injury is induced by interactions within a complex network of cytokines instead of a single factor. These cytokines could be mediators induced directly by burns, or secondary mediators, or could merely be markers of systemic inflammation or other concomitant injury, and it is not currently possible to distinguish between these possibilities. Further studies are required to identify which cytokines primarily increase, and which secondarily increase, after burn injury.
In conclusion, in this study, we have followed the changes in pro-inflammatory and anti-inflammatory cytokines for 3 weeks after burn injury in a Korean adult population. Our results suggest that IL-6, IL-8, IL-10, TNF-α, and G-CSF are important mediators in inflammatory change after burn injury, and that various factors including burn size may influence the concentrations of these cytokines in Korean adult burn patients.
Acknowledgements
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health, Welfare & Family Affairs, Republic of Korea (A084589) and a grant from Hallym University Medical Center Research Fund (01-2010-11).
References
1. Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DM, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006; 26:13–19. PMID: 16783192.

2. Finnerty CC, Herndon DN, Chinkes DL, Jeschke MG. Serum cytokine differences in severely burned children with and without sepsis. Shock. 2007; 27:4–9. PMID: 17172973.

3. Finnerty CC, Jeschke MG, Herndon DN, Gamelli R, Gibran N, Klein M, et al. Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med. 2008; 14:553–560. PMID: 18548133.

4. Jeschke MG, Mlcak RP, Finnerty CC, Norbury WB, Gauglitz GG, Kulp GA, et al. Burn size determines the inflammatory and hypermetabolic response. Crit Care. 2007; 11:R90. PMID: 17716366.

5. Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS ONE. 2011; 6:e21245. PMID: 21789167.

6. Gauglitz GG, Finnerty CC, Herndon DN, Mlcak RP, Jeschke MG. Are serum cytokines early predictors for the outcome of burn patients with inhalation injuries who do not survive? Crit Care. 2008; 12:R81. PMID: 18564432.

7. de Bandt JP, Chollet-Martin S, Hernvann A, Lioret N, du Roure LD, Lim SK, et al. Cytokine response to burn injury: relationship with protein metabolism. J Trauma. 1994; 36:624–628. PMID: 8189461.
8. Kowal-Vern A, Walenga JM, Hoppensteadt D, Sharp-Pucci M, Gamelli RL. Interleukin-2 and interleukin-6 in relation to burn wound size in the acute phase of thermal injury. J Am Coll Surg. 1994; 178:357–362. PMID: 8149035.
9. Drost AC, Burleson DG, Cioffi WG, Jordan BS, Mason AD Jr, Pruitt BA Jr. Plasma cytokines following thermal injury and their relationship with patient mortality, burn size, and time postburn. J Trauma. 1993; 35:335–339. PMID: 8371288.

10. Ivanova M, Ruiqing J, Kawai S, Matsushita M, Ochiai N, Maruya E, et al. IL-6 SNP diversity among four ethnic groups as revealed by bead-based liquid array profiling. Int J Immunogenet. 2011; 38:17–20. PMID: 21199388.

11. Sengupta S, Farheen S, Mukherjee N, Majumder PP. Pattern of nucleotide sequence variation in ICAM1 and TNF genes in twelve ethnic groups of India: roles of demographic history and natural selection. J Genet. 2007; 86:225–239. PMID: 18305342.
12. Delaney NL, Esquenazi V, Lucas DP, Zachary AA, Leffell MS. TNF-alpha, TGF-beta, IL-10, IL-6, and INF-gamma alleles among African Americans and Cuban Americans. Report of the ASHI Minority Workshops: Part IV. Hum Immunol. 2004; 65:1413–1419. PMID: 15603866.
13. Coe CL, Love GD, Karasawa M, Kawakami N, Kitayama S, Markus HR, et al. Population differences in proinflammatory biology: Japanese have healthier profiles than Americans. Brain Behav Immun. 2011; 25:494–502. PMID: 21112385.

14. Menon R, Merialdi M, Lombardi SJ, Fortunato SJ. Differences in the placental membrane cytokine response: a possible explanation for the racial disparity in preterm birth. Am J Reprod Immunol. 2006; 56:112–118. PMID: 16836613.

15. Holm S. A simple sequentially rejective multiple test prodecure. Scand J Statist. 1979; 6:65–70.
16. Sack U, Burkhardt U, Borte M, Schädlich H, Berg K, Emmrich F. Age-dependent levels of select immunological mediators in sera of healthy children. Clin Diagn Lab Immunol. 1998; 5:28–32. PMID: 9455875.

17. Dehne MG, Sablotzki A, Hoffmann A, Muhling J, Dietrich FE, Hempelmann G. Alteration of acute phase reaction and cytokine production in patients following severe burn injury. Burns. 2002; 28:535–542. PMID: 12220910.
18. Huang L, Yao Y, Dong N, Yu Y, He L, Sheng Z. Association between regulatory T cell activity and sepsis and outcome of severely burned patients: a prospective, observational study. Crit Care. 2010; 14:R3. PMID: 20064232.

19. Marano MA, Fong Y, Moldawer LL, Wei H, Calvano SE, Tracey KJ, et al. Serum cachectin/tumor necrosis factor in critically ill patients with burns correlates with infection and mortality. Surg Gynecol Obstet. 1990; 170:32–38. PMID: 2294627.
20. Yeh FL, Lin WL, Shen HD. Changes in circulating levels of an anti-inflammatory cytokine interleukin 10 in burned patients. Burns. 2000; 26:454–459. PMID: 10812267.

21. Lantos J, Földi V, Roth E, Wéber G, Bogár L, Csontos C. Burn trauma induces early HMGB1 release in patients: its correlation with cytokines. Shock. 2010; 33:562–567. PMID: 19997053.
22. Ozbalkan Z, Aslar AK, Yildiz Y, Aksaray S. Investigation of the course of proinflammatory and anti-inflammatory cytokines after burn sepsis. Int J Clin Pract. 2004; 58:125–129. PMID: 15055859.

23. Friedland JS, Porter JC, Daryanani S, Bland JM, Screaton NJ, Vesely MJ, et al. Plasma proinflammotory cytokine concentrations, Acute Physiology and Chronic Health Evaluation (APACHE) III scores and survival in patients in an intensive care unit. Crit Care Med. 1996; 24:1775–1781. PMID: 8917024.
Fig. 1
IL-6 (A), IL-8 (B), IL-10 (C), TNF-α (D), and G-CSF (E) concentrations according to postburn time and burn sizes. Data are presented as median ± interquartile range. Solid lines and • indicate medians of all 3 groups at each time point.
*Significant difference between Group I and Group II; †significant difference between Group II and Group III; ‡significant difference between Group I and Group III at each time point by Kruskal-Wallis test and post-hoc analysis.
Abbreviations: IL, interleukin; TNF, tumor necrosis factor; G-CSF, granulocyte-colony stimulating factor.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download