Abstract
Anaphylactic transfusion reactions are rare complications of blood transfusions. Anhaptoglobinemia, a condition that has high incidence in Asia, can cause allergic transfusion reactions or anaphylaxis in severe cases. A 50-yr-old Korean woman was diagnosed with relapsed acute promyelocytic leukemia. She developed thrombocytopenia during chemotherapy and an anaphylactic transfusion reaction on the 4th and 5th platelet transfusions immediately after the transfusion of the platelet concentrates was initiated. Blood analysis showed no detectable serum haptoglobin. We examined her genetic phenotype and detected anhaptoglobinemia, which occurs because of an allelic deletion in the Hp gene cluster. The presence of an antibody against haptoglobin was detected by performing ELISA. To prevent anaphylactic reactions, apheresis platelets were transfused after washing. Consequently, anaphylactic transfusion reactions did not develop. Here, we report the first case of anhaptoglobinemia causing anaphylactic transfusion reaction in Korea.
Allergic transfusion reactions (ATRs) are the most common complications of blood transfusions. ATRs present with mild to severe anaphylactic reactions and lead to dyspnea, shock, loss of consciousness, tachycardia, and in rare cases, death. Patients with IgA deficiency, anhaptoglobinemia, and C3 and C4 deficiencies are at an increased risk of developing ATRs because of the antibodies present in their plasma [1].
Haptoglobin, synthesized by hepatocytes in the liver, is a Hb-binding glycoprotein in the plasma. The haptoglobin phenotype is determined by a pair of codominant alleles-Hp1 and Hp2. Hence, there are 3 common genetic haptoglobin phenotypes-Hp1 and Hp2 with a homozygous genotype; and Hp2-1 with a heterozygous genotype. The main function of haptoglobin is to prevent Hb leakage by binding to Hb [2]. Hypohaptoglobinemia and anhaptoglobinemia may be acquired: Reduced synthesis of haptoglobin due to liver dysfunction can be observed in cases of liver cirrhosis and hepatocellular carcinoma. Increased consumption of haptoglobin for the formation of haptoglobin-Hb complex can be observed in cases of severe hemolysis. However, in some cases, like in the case of genetic homozygotes with an allelic deletion in the Hp gene cluster (Hpdel), hypohaptoglobinemia and anhaptoglobinemia have a genetic basis [3]. Hp0Hp2 has also been observed to be associated with hypohaptoglobinemia. The incidence of anhaptoglobinemia due to Hpdel has been reported to be about 1/1,500 among Koreans [3].
We report the case of a patient who developed anaphylaxis after the transfusion of leukoreduced platelet concentrates (PCs). On the basis of the results of PCR, the patient was diagnosed with anhaptoglobinemia resulting from homozygous allelic Hpdel [4, 5]. Presence of antibody against haptoglobin was detected by performing ELISA. We concluded that the antibodies against haptoglobin led to the development of anaphylactic reaction in this patient. We employed washed platelets to prevent further anaphylactic transfusion reactions, as this had been performed and demonstrated to be effective in previous studies [1, 6].
The Case report was approved by the Institutional Review Board (IRB) of the Severance Hospital. A 50-yr-old Korean woman was admitted to the Severance Hospital because of easy bruising and sore throat on September 5, 2011. She had been diagnosed with acute promyelocytic leukemia 3 yr ago and was confirmed to be in complete remission after chemotherapy. And she did not have liver disease or a history of hemolysis. Hematological analysis performed on admission showed a white blood cell count of 1.01×109/L, Hb level of 12.9 g/dL, and a platelet count of 95×109/L. Bone marrow (BM) aspiration and biopsy findings showed that the BM was filled with leukemic promyelocytes with irregular nuclear shape, dispersed chromatin, coarse azurophilic granules, and occasional faggot cells. Chromosomal study of the BM showed that 14 out of 20 mitotic figures were 46, XX, -6, t(15;17)(q22;12),+mar, and 6 exhibited a normal female karyotype (46, XX). The PML/RARA gene rearrangement in the BM was positive. Therefore, she was diagnosed with relapsed acute promyelocytic leukemia. Chemotherapy with arsenic trioxide was initiated on September 7, 2011, and filtered PCs were transfused 4 times for moderate thrombocytopenia (<50×109/L) on days 2, 3, 5, and 9 after admission. No significant ATR was observed except for mild chest tightness on the 4th platelet transfusion.
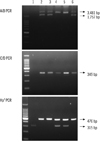
On the 14th day after admission, 1 unit of platelet apheresis was transfused because of severe thrombocytopenia (19×109/L) along with an anti-histamine medication. Immediately after the infusion, the patient developed dyspnea, urticaria, hypotension (systolic blood pressure, 84 mmHg and diastolic blood pressure, 36 mmHg), and fever (38.8℃). Discontinuation of the transfusion and injection of dexamethasone with 500 mL of normal saline resolved the anaphylaxis. After 2 days, her platelet count (11×109/L) decreased, and hence, 6 units of pooled leukoreduced PCs were transfused carefully along with an anti-histamine and dexamethasone. Although the patient was treated with premedication, the same anaphylactic reaction recurred. We surmised anhaptoglobinemia or IgA deficiency as the possible cause and analyzed her serum IgA and haptoglobulin levels. The level of IgA was 338 mg/dL, which is within the normal range, but serum haptoglobin was undetectable. We had adopted a haptoglobin genotyping method based on PCR. It is a simple method for detecting haptoglobin deletion by performing PCR. Primers A and B were used for amplifying the specific sequences of Hp1 and Hp2, and primers C and D were used for amplifying the Hp2 specific sequence. Primers Del-U and Del-L were used for amplifying the Hp0 allele. The haptoglobin genotype was found to be the Hpdel allele (Fig. 1), which causes Hpdel anhaptoglobinemia. Presence of an IgG antibody against the haptoglobin was detected by performing ELISA. In this patient, the anaphylactic reaction possibly occurred because of the presence of an alloantibody against the haptoglobin.
In order to prevent anaphylaxis, we decided to infuse the patient with washed and filtered PCs, which were prepared in the following manner: the filtered PCs were treated with 3 wash cycles involving plasma supernatant removal after dilution with 400 mL of 0.9% NaCl solution and centrifugation for 8 min at 261.75 rad/s in a Cobe 2991 Blood Cell Processor (Cardial BCT, Lakewood, Colorado, USA) [7]. With gentle manual rotation, the platelets in washed and filtered PCs were re-suspended and then placed on a washed and filtered PCs for a minimum of 30 min before using them for transfusion. The platelet recovery was over 80%. We did not observe anaphylactic reactions or ATRs on transfusing washed and filtered red blood cells .
Because anaphylactic transfusion reactions in their most severe forms can cause death, every effort should be made to prevent such catastrophic consequences. Previous studies have shown that transfusion reactions can be observed in patients with IgA deficiency, anhaptoglobinemia, and C3 and C4 deficiencies. This is due to the presence of antibodies against IgA, haptoglobin, and C3 and C4, resulting in the formation of antigen-antibody complexes [3, 8]. In Asia, the incidence of IgA deficiency (1/18,500) is lower than that of anhaptoglobinemia [9]. The incidence of anhaptoglobinemia is reported to be about 1/1,500 in Korea, 1/4,000 in Japan, and 1/1,000 in China [3]. Therefore, in Asia, paying attention to the possible causation of anhaptoglobinemia in anaphylactic transfusion reactions is of paramount importance. This report presents the first confirmed case of anaphylactic reaction in anhaptoglobinemia in a Hpdel homozygote in Korea.
Screening for the presence of serum haptoglobin antibodies should be performed in patients at high risk of developing genetic anhaptoglobinemia. A simple PCR method for detecting Hpdel is recommended to be a reliable method for preventing ATRs in genetically susceptible patients.
The transfusion of the plasma component present in the platelet product is responsible for the ATRs in these cases. Transfusion of washed platelets or platelets in platelet additive solution is an effective way of eliminating the plasma component [10]. Platelet washing can also be achieved by centrifugation and resuspension in saline without using a washing instrument such as Cobe 2991 (Cardial BCT). Plasma removal by the manual method is reported to result in less than 10% of loss of the platelets, and it does not affect the platelet function either [11]. Tobian et al. reported that washed platelets decrease the incidence of ATRs [1]. We also observed that ATRs could be prevented simply by transfusing washed platelets in our patient with anhaptoglobinemia.
The incidence of anhaptoglobinemia is higher in Korea than in the West. Hence, when ATRs are observed to occur in patients with anhaptoglobinemia, it is important to examine if antibody against haptoglobin is present and to conduct a genetic examination. Transfusion of washed platelets is a highly effective treatment for patients with anhaptoglobinemia who carry an antibody against haptoglobin.
Figures and Tables
Fig. 1
Determination of Hp1 and Hp2 alleles and haptoglobin gene deletion from the patient and control individuals in PCR. Only a 315 bp band was amplified from the patient with homozygous for the Hp0 allele. Lane 1, patient; lane 2, Hp2/Hp1 heterozygote; lane 3, Hp2/Hp1 heterozygote; lane 4, Hp2/Hp0 heterozygote; lane 5, Hp2/Hp0 heterozygote; lane 6, Hp2/Hp1 heterozygote.
Acknowledgement
We acknowledge all the staff members of blood bank and immunoserology in the Severance Hospital.
References
1. Tobian AA, Savage WJ, Tisch DJ, Thoman S, King KE, Ness PM. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion. 2011. 51:1676–1683.

2. Levy AP, Asleh R, Blum S, Levy NS, Miller-Lotan R, Kalet-Litman S, et al. Haptoglobin: basic and clinical aspects. Antioxid Redox Signal. 2010. 12:293–304.

3. Koda Y, Watanabe Y, Soejima M, Shimada E, Nishimura M, Morishita K, et al. Simple PCR detection of haptoglobin gene deletion in anhaptoglobinemic patients with antihaptoglobin antibody that causes anaphylactic transfusion reactions. Blood. 2000. 95:1138–1143.

4. Park KU, Song J, Kim JQ. Haptoglobin genotypic distribution (including Hp0 allele) and associated serum haptoglobin concentrations in Koreans. J Clin Pathol. 2004. 57:1094–1095.

5. Koda Y, Soejima M, Yoshioka N, Kimura H. The haptoglobin-gene deletion responsible for anhaptoglobinemia. Am J Hum Genet. 1998. 62:245–252.

6. Vo TD, Cowles J, Heal JM, Blumberg N. Platelet washing to prevent recurrent febrile reactions to leucocyte-reduced transfusions. Transfus Med. 2001. 11:45–47.

7. Vesilind GW, Simpson MB, Shifman MA, Colman RE, Kao KJ. Evaluation of a centrifugal blood cell processor for washing platelet concentrates. Transfusion. 1988. 28:46–51.

8. Nishiki S, Hino M, Kumura T, Hashimoto S, Ohta K, Yamane T, et al. Effectiveness of washed platelet concentrate and red cell transfusions for a patient with anhaptoglobinemia with antihaptoglobin antibody. Transfus Med. 2002. 12:71–73.

9. Mantovani AP, Monclaro MP, Skare TL. Prevalence of IgA deficiency in adult systemic lupus erythematosus and the study of the association with its clinical and autoantibody profiles. Rev Bras Reumatol. 2010. 50:273–282.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download