Abstract
Background
We investigated the influence of pre-analytical factors on the results of clinical tests and thereby analyzed approaches to improve quality management in clinical laboratories.
Methods
Unqualified clinical samples were selected from all the samples received at our clinical laboratory. The data were collected for 2009 and 2010, i.e., the years before and after the establishment of the laboratory quality management system. The rate and causes of generation of unqualified samples were analyzed, and measures to improve the laboratory practices were studied and implemented.
Results
A total of 1,051 unqualified samples were identified from among the 553,158 samples (the overall incidence rate of unqualified samples was 0.19%). The number of unqualified samples substantially varied according to the nature of the sample, and clinical samples collected for routine blood tests or coagulation tests were the predominant unqualified samples. The main causes of generation of unqualified samples were insufficient sample volumes and improper methods of mixing the samples. The rate of generation of unqualified samples decreased significantly after the implementation of improvement measures (0.26% in 2009 vs. 0.13% in 2010, P<0.001).
Conclusions
The number of unqualified samples decreased significantly after the establishment of the laboratory quality management system, which promoted active communication among and training of the clinical staff to reduce the occurrence of pre-analytical errors. Comprehensive control of pre-analytical factors is an important approach in improving the clinical laboratory practices.
In the recent years, the importance of transparency in customer service and organizational administration has grown in many fields, including administration of medical institutions. Ensuring proper communication with patients and transparency in medical activities have become essential in this regard. International Organization for Standardization (ISO) 15189, which specifically applies to clinical laboratories, is an international standard issued in 2003. This standard has been commonly employed as an accreditation tool for clinical laboratories in China, Europe, Australia, Japan, etc. Quality programs in clinical laboratories traditionally focused on the processes under direct control of the laboratories, particularly in the analytical phase. However, most errors occur during pre- and post-analytical phases [1-4], which reflects the requirement of extensive quality control in these phases.
Since errors and problems are prevalent in the pre-analytical phase of diagnostics, this phase is the most critical phase with much scope for improvement. However, there have been few improvements in this phase, and some testing errors occur in the pre-analytical phase [5]. Therefore, reducing the number of unqualified samples would contribute to quality management in clinical laboratories. The most effective way to avoid any other type of medical errors is to implement a complete quality management system. We recognized the difficulty in controlling errors in the pre-analytical phase during our preparation for the surveillance tests for ISO15189 accreditation. Therefore, in this study, we analyzed the reasons for these errors and identified effective measures that would lead to significant improvements in the clinical laboratory quality management system and in clinical laboratory practices.
The ISO15189: Pre-examination procedures include the requisites for manual collection of primary samples, traceability of the primary samples to an identifiable individual, sample monitoring during transport, recording of receipts of samples, and processing of urgent samples [6, 7]. In the study, we defined unqualified samples on the basis of some critical analytical activities, such as the accuracy of patient identification, incorrect sample-collection procedures (for example, use of unsuitable samples for microbiologial analysis and hemolysis and clotting of blood samples), and insufficient sample volume. The use of inappropriate containers was observed to be particularly high for inpatient samples, and this was observed to yield unqualified samples in the pre-analytical phase [8-10]. The study was conducted according to a format, including the reason of unqualified samples, information on the clinical department that collected the sample, and information on the nurse who received the unqualified samples. The laboratory information system (LIS) automatically recorded the data for the total number of samples collected at our clinical laboratory. Information about the unqualified samples was recorded by laboratory technicians who were usually in charge of sample collection. We obtained all the information and records for the period from 2009 to 2010.
A descriptive study was performed using 1,051 unqualified samples obtained at the department of laboratory medicine in a teaching hospital between 2009 and 2010. The data collection forms were created in January 2010, and the modified form was used for 11 months (from January 2010 to December 2010) to collect the baseline data of samples that were processed completely in the clinical laboratory. All the data were analyzed using the Statistical Package for the Social Sciences software and the χ2 test.
The unqualified samples were classified on the basis of the nature of the sample, causes of disqualification, and the departments sending the samples. We analyzed the monthly rate of generation of unqualified samples from January 2010. We then uploaded this data on the website of the hospital so that the clinical departments could access it every month. We observed which department sent the most unqualified samples in a certain month and accordingly planned training programs for the nurses of that department. We met the clinical staff regularly to ensure timely and efficient communication with the staff and discussed and created appropriate training programs for them.
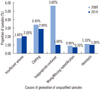
We plotted the data for these 2 yr on a chart. The chart showed an overall substantial decrease in the processing time for samples at our clinical laboratory (Fig. 1). We observed a significant difference in the rate of generation of unqualified samples between 2009 and 2010 (P<0.05). A total of 1,051 unqualified samples were collected (603 intervention-group samples in 2009 and 448 control-group samples in 2010). The total rate of generation of unqualified samples was 0.26% in 2009 and 0.13% in 2010 (P<0.001). The most frequent cause of generation of unqualified samples was improper sample collection in microbiological analysis (1.01%), complete blood count (0.38%), and coagulation analysis (0.57%) (Table 1). In both the intervention and control groups, the following factors were responsible for the generation of unqualified samples: hemolysis, clotting of blood samples collected for complete blood count analysis, insufficient sample volume, or improper sample collection methods (Fig. 2). The results of the χ2 test showed that the rate of generation of each type of unqualified sample was significantly lower in 2010 than in 2009 (P<0.001). Thus, improper sample collection played a major role in the generation of unqualified samples.
In the age of evidence-based medicine, the results of clinical laboratory testing are integral to clinical decision-making and facilitate diagnosis and monitoring of therapy and prediction of the clinical outcome. Because of the increasing demands for laboratory assessments and the requirement of standardization in clinical laboratories, the primary goal should be to ensure highly efficient communication between the clinical staff and patients. The balance between efficiency and quality is emerging as a strategic goal. Since the initiation of accreditation procedures, quality control measures are being gradually established in clinical laboratories around the world. Laboratory technicians have strived to make diagnostic practices safe, effective, patient-centered, timely, and fair; decreasing the errors in clinical laboratory practices is one of the most important factors in achieving these objectives [11-15].
Lundberg's brain-to-brain loop provides a comprehensive representation of the entire testing process, which is divided into pre-analytical, analytical, and post-analytical phases [15]. The clinical laboratories alone are responsible for the analytical phase, whereas the pre-analytical phase involves laboratories, clinicians, patients, and many other factors associated with data entry, specimen collection, and transport. Previous studies have systematically addressed many issues associated with pre-analytical variability, such as patient identification, specimen rejection, and contamination of blood/urine/sputum/stool culture. In order to achieve improvements in quality and reduce errors, it is necessary to study the pre-analytical process as a whole. To this end, clinical laboratories should map the pre-analytical phase in its entirety, identify factors that can cause unnecessary variability and lead to errors in laboratory tests, and finally, find ways to either remove or correct these errors. Significant improvements in quality management can be achieved through efforts directed at the phases described above (Fig. 1, Table 1).
In our laboratory, the review of unqualified samples in 2009 recorded on special forms helped detect the incidence rate of sample rejection. It must have been rather difficult for the laboratory staff and clinicians to promptly identify the source of these unexpected results, and misinterpretation would have had a considerable impact on the well-being of the patients [16]. Therefore, in January 2009, we initiated a monthly program for monitoring the unqualified samples, and analyzed the causes for generation of these samples. In early 2009, we sent the monthly information to every clinical department through the hospital website. The clinical laboratory communicated with the head nurse of the department showing the most number of unqualified samples, and helped the nurses and workers or even the physicians obtain the samples in a correct way. In addition, an education group comprising the laboratory staff from each subdivision started to train the individuals involved in collection and transmission of the samples [17]. Simultaneously, we also established the importance of timely and effective communication with the clinical staff, which helps reduce unnecessary errors and obtain the required process efficiency. The rates of generation of unqualified samples decreased from 0.26% in 2009 to 0.13% in 2010, and the lowest rate was observed in May and October in the 2 yr (Fig. 1). We train the new employees every May or October, which showed a reduction in the rate of generation of unqualified samples in the 2 yr.
The main reasons for generation of unqualified samples have been described previously (Fig. 2). The most common reasons were insufficient sample volume and clotting of the samples. To avoid such situations during sample collection, constant training of clinical nurses is necessary. Therefore, continuous improvements in the pre-analytical phase are very essential.
In conclusion, long-term monitoring of unqualified samples as performed in our study will result in improvements in the laboratory practices and reduction in the rate of generation of unqualified samples. Monitoring programs in all the areas of clinical laboratory will help maintain the quality standards in laboratory practices.
Figures and Tables
References
1. Stankovic AK. The laboratory is a key partner in assuring patient safety. Clin Lab Med. 2004. 24:1023–1035.

2. Rivers PA, Dobalian A, Germinario FA. A review and analysis of the clinical laboratory improvement amendment of 1988: compliance plans and enforcement policy. Health Care Manage Rev. 2005. 30:93–102.
3. Johnson PR. The contribution of proficiency testing to improving laboratory performance and ensuring quality patient care. Clin Leadersh Manag Rev. 2004. 18:335–341.
4. Howanitz PJ. Errors in laboratory medicine: practical lessons to improve patient safety. Arch Pathol Lab Med. 2005. 129:1252–1261.

5. Bonini P, Plebani M, Ceriotti F, Ceriotti F. Errors in laboratory medicine. Clin Chem. 2002. 48:691–698.

6. Plebani M. Appropriateness in programs for continuous quality improvement in clinical laboratories. Clin Chim Acta. 2003. 333:131–139.

7. Plebani M, Carraro P. Mistakes in a stat laboratory: types and frequency. Clin Chem. 1997. 43:1348–1351.

8. Lippi G, Bassi A, Brocco G, Montagnana M, Salvagno GL, Guidi GC. Preanalytic error tracking in a laboratory medicine department: results of a 1-year experience. Clin Chem. 2006. 52:1442–1443.

9. Howanitz PJ. Errors in laboratory medicine: practical lessons to improve patient safety. Arch Pathol Lab Med. 2005. 129:1252–1261.

10. Saxena S, Kempf R, Wilcox S, Shulman IA, Wong L, Cunningham G, et al. Critical laboratory value notification: a failure mode effects and criticality analysis. Jt Comm J Qual Patient Saf. 2005. 31:495–506.

11. Ricós C, Garcia-Victoria M, de la Fuente B. Quality indicators and specifications for the extra-analytical phases in clinical laboratory management. Clin Chem Lab Med. 2004. 42:578–582.

12. Barenfanger J, Sautter RL, Lang DL, Collins SM, Hacek DM, Peterson LR, Improving patient. read-back) telephone reports of critical information. Am J Clin Pathol. 2004. 121:801–803.
13. Berte LM. Patient safety: getting there from here-quality management is the best patient safety program. Clin Leadersh Manag Rev. 2004. 18:311–315.
14. Bachner P. Quality assurance of the analytic process: pre- and postanalytic variation. Clin Lab Med. 1986. 6:613–623.

15. Lundberg GD. How clinicians should use the diagnostic laboratory in a changing medical world. Clin Chim Acta. 1999. 280:3–11.

16. Coiera E. Communication systems in healthcare. Clin Biochem Rev. 2006. 27:89–98.
17. Rai AJ, Vitzthum F. Effects of preanalytical variables on peptide and protein measurement in human serum and plasma; implication of clinical proteomics. Expert Rev Proteomics. 2006. 3:409–426.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download