Abstract
Background
Studies that evaluate the effect of age on stone composition are scarce. The aim of this study was to highlight the changes in epidemiological characteristics (stone composition and location) of urolithiasis according to patients' age.
Methods
We studied 1,301 urolithiasis patients with age ranging from 6 months to 92 yr (781 males and 520 females). Stone analysis was performed using a stereomicroscope and infrared spectroscopy to determine the morphological type and molecular composition of each stone.
Results
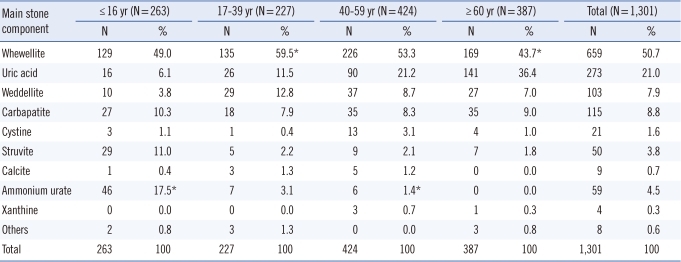
The annual average incidence of new stone formation was 31.7 per 100,000 persons. In 71.8% of cases, calculi were located in the upper urinary tract. Compared to other age groups, children and old men were more affected by bladder stones. Calcium oxalate monohydrate was the most frequent stone component, even though its frequency decreased with age (59.5% in young adults and 43.7% in the elderly, P<0.05) in favor of an increase in uric acid stones (11.5% in young adults and 36.4% in the elderly, P<0.05). Struvite stones were rare (3.8%) and more frequent in children than in adults.
Go to : 
Urolithiasis is a relatively common condition in different continents and countries. Nevertheless, the overall probability of forming stones considerably differs in various parts of the world [1, 2]. The risk of developing urolithiasis appears to be higher in the western hemisphere than in the eastern hemisphere, even though the highest risks have been reported in some Asian countries [2, 3]. Socioeconomic conditions have generated changes in the incidence, location, and composition of urolithiasis [4]. In fact, bladder stones composed of ammonium urate and calcium oxalate have been reported to be endemic in Asia, whereas renoureteral calculosis, featuring mainly calcium oxalate and phosphate, is currently more frequent in economically developed countries [1]. This must be attributed to the different lifestyles and dietary habits.
Because of the continuous changes in lifestyles and dietary habits, the relationships between age and composition can also change with time [5]. Only a few reports to date have suggested a relationship between stone composition and patient's age [6-8]. In 1995, Daudon et al. [9] reported an increase in the frequency of uric acid stones with an increase in patient's age; they reported that the frequency reaches its peak in the age range 60-70 yr. In Japan, Koide et al. [8] have reported that the peak frequency of calcium oxalate urolithiasis was observed between 40 and 50 yr of age.
Urolithiasis in developing countries was considered very different from urolithiasis observed in industrialized countries [10]. In Tunisia, studies evaluating the epidemiological characteristics (stone composition and location) of urolithiasis are scarce and have been based on the analysis of a limited number of patients [6]. This paper reports pioneering research in which we examined 1,301 lithiasic patients for 10 yr in order to present an outline of the current state of renal lithiasis on the Tunisian coast region.
Go to : 
Between July 1998 and September 2010, we retrospectively investigated 1,301 patients with stones and who were admitted to the pediatric and the urologic surgery departments at the University Hospital of Monastir. The patients, 781 males and 520 females, were aged 6 months to 92 yr. To make our task easier, we divided our patients into 4 age groups according to the Tunisian classification: children, young adults, adults, and elderly.
Full documentation included recording of age, sex, residency, age at onset of symptoms, age at diagnosis of stone disease, clinical presentation, past medical and surgical history, family history of stone disease, and recurrence (which was considered if the patient had previous surgery or spontaneous passage of a stone before presentation). Urine culture was carried out in 987 cases.
Twenty-four-hour urine determination of urinary calcium, oxalate, and uric acid was performed for all patients. Hypercalciuria (HCa) was defined as urine calcium excretion >4 mg/(kg 24 hr) [11]. Hyperoxaluria (HOx) was defined as urine oxalate excretion >55 mg/(1.73 m2 24 hr). Hyperuricosuria (HUr) was defined as uric acid excretion >815 mg/(1.73 m2 24 hr) [12].
When possible, the structure of each stone was identified using a stereomicroscope to define the morphology of the stone and to select its representative parts (nucleus or core, internal section, and external surface) and using infrared spectroscopy to determine its molecular and crystalline composition. These techniques were used to analyze all stones collected during the 10-yr study period. Approximately 0.5 to 2 mg of powder was collected from each part of the stone and pulverized with an inert powdered support (dried potassium bromide) in a proportion of 0.5-2% in an agate mortar. This mixture was transferred into an appropriate container and pressed at 10 t/cm2 to form a transparent pellet with a diameter of 13 mm. The spectral region investigated was from 4,000 to 400 per cm. Reference spectra were obtained from pure potassium bromide (KBr) pellets. The spectra were recorded by means of a Bruker IFS25 Fourier transform infrared spectrometer (Bruker SA, Wissembourg, France). A global powder of the sample was analyzed in order to quantify the relative proportions of the various stone components. Only the qualitative and quantitative composition obtained from the whole-stone powder constituted the study material in this report.
The various compounds were identified by comparison to previously published reference spectra. The results were expressed according to the main crystalline phase found in the stones and were named as follows: whewellite (calcium oxalate monohydrate), weddellite (calcium oxalate dihydrate), carbapatite (carbonated calcium phosphate crystallized in a hexagonal pattern), struvite (magnesium ammonium phosphate hexahydrate), and calcite (anhydrous calcium carbonate). The stone component was considered the main element if it exceeded 75% of the total composition of the calculus. Stones formed by a single component were classified as pure stones, and those with more than one component were classified as mixed stones. Statistical analysis of the data was performed using SPSS 11.0 for Windows. Statistical significance was determined using the χ2-test. P values less than 0.05 were considered significant.
Go to : 
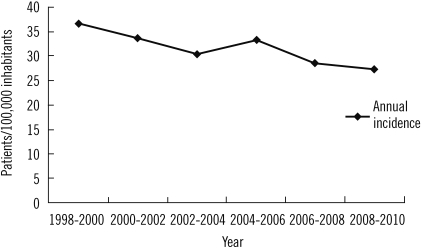
The average annual incidence of new stone formation was 31.7 per 100,000 inhabitants. The annual incidence was 7.4 per 100,000 inhabitants for the pediatric age group and 54.5 per 100,000 inhabitants for the adult age group. The average annual incidence decreased during the last year of our study (Fig. 1). The most frequent method of extraction was conventional surgery (92.2%). The other methods included extracorporeal lithotripsy (6.2%), spontaneous passage (1.3%), and endoscopy (0.3%).
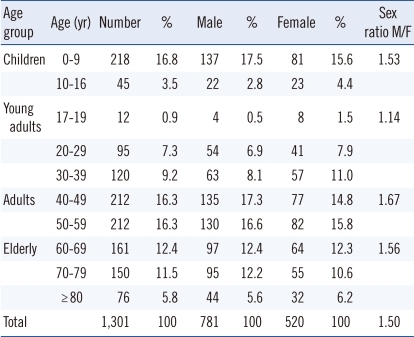
Of the 1,301 patients with urolithiasis, 781 (60%) were males and 520 (40%) were females with a sex ratio of 1.5:1. Patient's age at presentation ranged from 6 months to 92 yr. The number of patients stratified by age and sex is shown in Table 1. The highest number of calculi in males and females was observed in the age groups 40-49 and 50-59 yr, respectively.
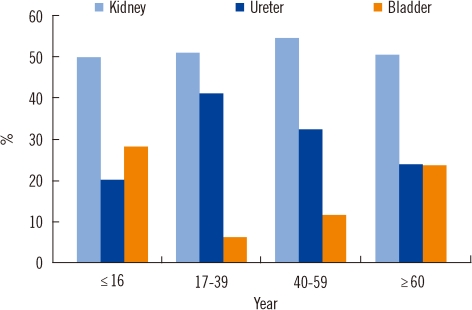
The upper urinary tract was most frequently affected by this condition (kidney 52.4% and ureter 29.4%). Bladder stones were noted in 18.2% of cases. A significant difference in bladder stone cases, was noted according to gender (63.2% in male vs. 36.8% in female) (P<0.001).
Children and elderly patients seemed to have a higher incidence of lower urinary tract stones (Fig. 2). A male predominance was pronounced in children (M/F=2.86). Prostate hyperplasia was associated with bladder stone findings in 12 elderly male patients.
A family history of stone disease was recorded for 54 patients (4.1%). The most common symptom on admission was abdominal pain in 39% of cases. This was accompanied by colic pain in 28.2%, hematuria in 11.7%, dysuria in 9.1%, urinary tract infection in 3.2%, anuria in 2.8%, accidental finding in 2.8%, fever in 1.9%, and dumb kidney in 1.3%.
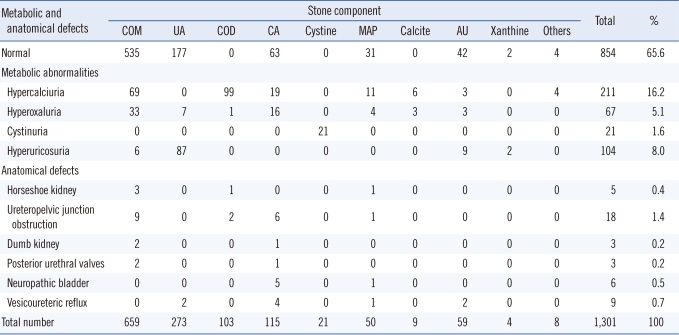
Anatomical defects were detected in 44 patients (Table 2) and were associated with urinary tract infection in only 11 cases. Urinary stasis secondary to a urinary tract anomaly was noted in 8 patients (18%). Metabolic disorders (confirmed by 24-hr urine collection) were observed in 403 patients (31.0%). Two hundred and eleven patients had severe hypercalciuria, 67 had hyperoxaluria, and 21 had cystinuria (Table 2). Hyperuricosuria, detected in 104 patients, was the second most common metabolic disorder and was more frequent in the elderly.
As shown in Table 3, the most frequent component of urinary stones, as determined by infrared spectroscopy, was whewellite (50.7%). However, its frequency differed with age. Whewellite was significantly more frequent in young adults (59.5%) than in the elderly (43.7%) (P<0.05). The proportion of stones with uric acid was 21.0%, and such stones were more prevalent in the elderly than in the other age groups (P<0.02). However, ammonium urate stones were predominant in children (P<0.001). Struvite-containing stones were observed in 3.8% of cases and were more frequent in children than the other age groups (P<0.02).
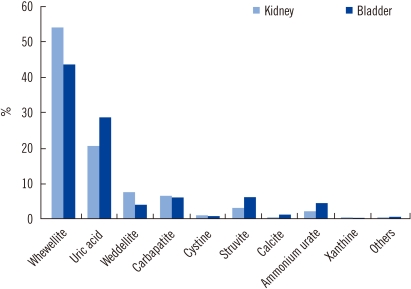
According to stone location, struvite stones were more frequently observed in the bladder (6.8%) than in the kidneys (3.7%), whereas whewellite stones were more frequently found in the kidneys (54.1% vs. 44.1% in the bladder) (Fig. 3). Ammonium urate was observed in 5.1% of bladder stones, and it was noted only in children (15.8%).
The treatment procedures performed were as follows: 1) open surgical procedure was performed in 1,057 patients (81.2%); 2) stones were disintegrated with extracorporeal shock wave lithotripsy in 185 patients (14.2%); 3) endoscopic interventions (simple forceps and/or basket extraction) were used in 22 patients (1.7%); and 4) a specific treatment with D-penicillamine (urine pH in the low alkaline range) was used in 21 cystinuria patients. However, treatment with D-penicillamine was successful in only 14 patients. The remaining 23 patients (1.8%) required no specific intervention, and the majority passed the stones spontaneously with conservative management and close follow-up.
Of the 1,301 patients, 288 (22.1%) experienced recurrences since an initial diagnosis of stone disease. The incidence of stone recurrence in patients who were radiographically stone free following ESWL was reported as 40.0% (74 cases). Disease recurrence occurred at 5 yr after the initial diagnosis based on the probable criteria.
Go to : 
Changes in socioeconomic conditions have generated changes in the incidence and type of urolithiasis in terms of both the site and the physicochemical composition of the calculi. Major variations in worldwide occurrence of urolithiasis have been reported according to geographical areas [10]. We found that the annual incidence of new stone formation in Tunisian patients was 31.7 per 100,000 Tunisians. This rate is similar to that reported in England (22 per 100,000 inhabitants) [13] and Kuwait (23.9 per 100,000 inhabitants). However, it is significantly different from that reported in Sweden (140 per 100,000 inhabitants) [14], Italy (168 per 100,000 inhabitants) [15], and the USA (277 per 100,000 inhabitants) [16]. In Europe, urinary stones are mainly located in the upper urinary tract, and the proportion of bladder calculi does not exceed 10.0% [10, 17]. Bladder stones have also been found to be frequent in the elderly as reported by some studies [18] but not in others [17]. According to Daudon et al. [18], 40.0% of the patients they analyzed were men over 80 yr. In our study, 18.2% of the stones were from the bladder, and elderly men were the most affected (24.3%). Prostatic hyperplasia, which is considered a frequent cause of bladder outlet obstruction, is frequent in old men and could be a possible explanation for the high frequency of bladder stones in the elderly [18, 19]. Females were also exposed to lower urinary tract stones (36.8%). This suggests that other risk factors, such as changes in bladder function associated with relaxation of smooth muscle tonicity in the elderly, reduce the efficiency of bladder emptying and promote urine stasis [18].
The pattern of urolithiasis in adult patients in Tunisia is not different from that observed worldwide. Stone composition has changed substantially over the past decades with a progressive increase in the frequency of calcium oxalate stones, which represents 70-80% of stones [20]. According to Daudon et al. [21], calcium oxalate stones in developing countries are mainly observed in North Africa and Asia Minor. In our study, calcium oxalate stones were found in 58.6% of all cases. This rate is comparable to those reported in French [9], Brazilian [22], and Spanish [5] papers. As recognized worldwide, hypercalciuria was the most frequent underlying factor in calcium oxalate stones, even though in some countries in the eastern hemisphere, hypocitraturia has been reported as the leading cause [23]. Daudon et al. [7] used a multivariate approach based on correspondence factor analysis and confirmed the relationship between stone composition and patient age. However, some variations according to geographical areas have been reported. In 1993, Baker et al. [24] reported that in Australia, the peak frequency of calcium oxalate urolithiasis was observed in persons between 50 and 60 yr of age. In Europe, studies had revealed that calcium oxalate stones are more frequent in persons between 40 and 50 yr of age [5, 7]. In Asia, it seems that the peak frequency of calcium oxalate stones occurs at an earlier age (30-50 yr) [8]. In our study, we found a peak frequency of calcium oxalate urolithiasis in persons between 16 and 39 yr of age; similar results have also been observed in Algeria [25]. The monohydrate form (whewellite) was 6.5 times more abundant than the dihydrate form (weddellite). Weddellite calculi occurred earlier in female patients than in male patients and decreased with increasing age; this finding is similar to that of Algerian [25] and French [9] studies. Abrams [26] has reported that both vitamin D deficiency and a diminished ability to absorb dietary calcium are more prevalent in the elderly. This could explain, in part, the decrease of weddellite and whewellite frequency in older individuals. In fact, a greater proportion of dietary calcium remains in the intestine where it is available to bind oxalate, thereby reducing oxalate absorption and the subsequent concentration of urinary oxalate [27].
Chronic diarrhea with subsequent acidosis during the first months of life and simultaneous phosphorus deficiency can explain a high urine excretion of ammonium ions. Ammonium ions can form urate ammonium by combining with urate ions, which are also abundant in child urine. In our series, ammonium urate was observed as often in kidney stones as in bladder stones; however, a high proportion (15.8%) of pediatric bladder stones was nucleated on ammonium urate. This suggests that hyperuricosuria, low phosphorus intake, low diuresis, and chronic diarrhea in children may be factors affecting stone formation.
Struvite is the best marker of urinary tract infections by urease-producing bacteria. Our study found a relatively lower rate of struvite stones (3.8%) than other stones; this rate was similar to the rates reported in Europe [7, 28], Algeria [24], and China [29]. This infection lithiasis frequency was lower than that reported by Najjar et al. [30] in the same area. Antibiotics abuse may be one reason for the low rate of infection lithiasis in developed countries [29]. However, we think the earlier detection of urinary infections and the greater attention paid to their treatment in recent years could explain the lower frequency in Tunisia. Contrary to the findings of other studies in France [7, 9], Algeria [25], and China [29], we found that uric acid stone was a very common type of stone disease in our region, accounting for 21.0% of cases. However, this frequency was similar to those reported in Germany [31], the USA [32], and Australia [24].
Hyperuricosuria and low urinary pH (below 5.5) seem to be the most important risk factors for uric acid crystallization [33]. In our series, hyperuricosuria was reported in only 107 cases, thereby suggesting the presence of others risk factors such as dietary habits. Indeed, since the 1960s, these habits have changed in our country towards an increase of purine-rich food (animal protein and seafood) intake [34], which explains the associated increase in uric acid excretion. As reported in industrialized countries [5, 7-9, 24], our data shows a clear increase of uric acid stones according to age in both genders. On the basis of the well-established pH dependence of uric acid urolithiasis, the rising proportion of uric acid stones with increasing age may be due to a progressive defect in urine ammoniagenesis that manifests with aging [35]. Daudon et al. [36] have shown that diabetic patients are more likely affected by uric acid stones. In addition, recent studies have suggested that the increased prevalence of urolithiasis and recurrence is associated with obesity and elevated urinary excretion of calcium, uric acid, and oxalate [37]. We now know that overeating contributes to the development of obesity and is strongly involved in the development of diabetes, kidney stones, and hypertension. These risk factors, such as obesity, diabetes, and hypertension, should be considered in epidemiological investigations in order to avoid confusion with the influence of age on stone composition and in order to have representative results.
Some studies have shown stone recurrence rates between 26% and 53% after 10 yr [38]. Previous ESWL has been found to be a risk factor in some studies [39] but not in others [40]. In our study, we report a recurrence rate, but the implication of ESWL as a risk factor remains to be confirmed.
In conclusion, although data on kidney stone disease in Tunisia are scarce, our data provide an idea about its epidemiologic characteristics. Calcium oxalate stones remain the most frequent components in all age groups, even if their frequency decreases in the elderly. As reported by other studies, we found that uric acid stones increase with increasing age, and infection stones are rarely observed in adults. Analysis of these data showed that urinary stones seen in Tunisian patients are tending to evolve in the same direction as stones seen in patients in industrialized countries.
Go to : 
Acknowledgement
The authors wish to thank Professor Walid Chaouachi for his assistance with the English-language presentation of the manuscript.
Go to : 
References
1. Trinchieri A. Epidemiology of urolithiasis. Arch Ital Urol Androl. 1996; 68:203–249. PMID: 8936716.
2. Ramello A, Vitale C, Marangella M. Epidemiology of nephrolithiasis. J Nephrol. 2000; 13(Suppl 3):S45–S50. PMID: 11132032.
3. Robertson WG, Hughes H. Ryall R, Bais R, editors. Epidemiology of urinary stone disease in Saudi Arabia. Urolithiasis 2. 1994. New York London: Plenum Press;p. 453–455.

4. El-Reshaid K, Mughal H, Kapoor M. Epidemiological profile, mineral metabolic pattern and crystallographic analysis of urolithiasis in Kuwait. Eur J Epidemiol. 1997; 13:229–234. PMID: 9085010.
5. Costa-Bauzá A, Ramis M, Montesinos V, Grases F, Conte A, Pizá P, et al. Type of renal calculi: variation with age and sex. World J Urol. 2007; 25:415–421. PMID: 17525848.

6. Alaya A, Najjar MF, Nouri A. Changes in stone composition according to age in Tunisian pediatric patients. Int Urol Nephrol. 2010; 42:621–628. PMID: 19937117.

7. Daudon M, Doré JC, Jungers P, Lacour B. Changes in stone composition according to age and gender of patients: a multivariate epidemiological approach. Urol Res. 2004; 32:241–247. PMID: 15127165.

8. Koide T, Itatani H, Yoshioka T, Ito H, Namiki M, Nakano E, et al. Clinical manifestations of calcium oxalate monohydrate and dihydrate urolithiasis. J Urol. 1982; 127:1067–1069. PMID: 7087010.

9. Daudon M, Donsimoni R, Hennequin C, Fellahi S, Le Moel G, Paris M, et al. Sex- and age-related composition of 10 617 calculi analyzed by infrared spectroscopy. Urol Res. 1995; 23:319–326. PMID: 8839389.

10. López M, Hoppe B. History, epidemiology and regional diversities of urolithiasis. Pediatr Nephrol. 2010; 25:49–59. PMID: 21476230.

11. Elder JS. Behrman RE, Kliegman RM, editors. Urinary lithiasis. Nelson textbook of pediatrics. 2004. 17th ed. Philadelphia: Saunders;p. 1822–1826.

12. Oner A, Demircin G, Ipekçioğlu H, Bülbül M, Ecin N. Etiological and clinical patterns of urolithiasis in Turkish children. Eur Urol. 1997; 31:453–458. PMID: 9187907.

13. Power C, Barker DJ, Blacklock NJ. Incidence of renal stones in 18 British towns. A collaborative study. Br J Urol. 1987; 59:105–110. PMID: 3828703.

14. Ahlstrand C, Tiselius HG. Renal stone disease in a Swedish district during one year. Scand J Urol Nephrol. 1981; 15:143–146. PMID: 7330608.

16. Curhan GC, Rimm EB, Willett WC, Stampfer MJ. Regional variation in nephrolithiasis incidence and prevelance among United States men. J Urol. 1994; 151:838–841. PMID: 8126805.
17. Neuzillet Y, Lechevallier E, Ballanger P, Ferriere JM, Saussine C, Doré B, et al. Urinary stones in subjects over the age of sixty. Prog Urol. 2004; 14:479–484. PMID: 15776895.
18. Daudon M. Évolution de la composition et de la localisation des calculs chez le sujet âgé. Feuillets de Biologie. 2003; 25:51–54.
19. El-Reshaid K, Mughal H, Kapoor M. Epidemiological profile, mineral metabolic pattern and crystallographic analysis of urolithiasis in Kuwait. Eur J Epidemiol. 1997; 13:229–234. PMID: 9085010.
20. Daudon M. Comment analyser un calcul et comment interpréter le résultat. L' Eurobiologiste. 1993; 203:35–46.
21. Daudon M, Bounxouei B, Santa Cruz F, Leite da Silva S, Diouf B, Angwafoo FF 3rd, et al. Composition of renal stones currently observed in non-industrialized countries. Prog Urol. 2004; 14:1151–1161. PMID: 15751409.
22. Silva SF, Matos DC, Silva SL, Daher Ede F, Campos Hde H, Silva CA. Chemical and morphological analysis of kidney stones: a double-blind comparative study. Acta Cir Bras. 2010; 25:444–448. PMID: 20877956.

23. Stitchantrakul W, Kochakarn W, Ruangraksa C, Domrongkitchaiporn S. Urinary risk factors for recurrent calcium stone formation in Thai stone formers. J Med Assoc Thai. 2007; 90:688–698. PMID: 17487123.
24. Baker PW, Coyle P, Bais R, Rofe AM. Influence of season, age, and sex on renal stone formation in South Australia. Med J Aust. 1993; 159:390–392. PMID: 8377690.

25. Djelloul Z, Djelloul A, Bedjaoui A, Kaid-Omar Z, Attar A, Daudon M, et al. Urinary stones in Western Algeria: study of the composition of 1,354 urinary stones in relation to their anatomical site and the age and gender of the patients. Prog Urol. 2006; 16:328–335. PMID: 16821346.
26. Abrams SA. Calcium turnover and nutrition through the life cycle. Proc Nutr Soc. 2001; 60:283–289. PMID: 11681644.
27. Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004; 15:3225–3232. PMID: 15579526.

28. Leusmann DB, Blaschke R, Schmandt W. Results of 5,035 stone analyses: a contribution to epidemiology of urinary stone disease. Scand J Urol Nephrol. 1990; 24:205–210. PMID: 2237297.

29. Sun X, Shen L, Cong X, Zhu H, He L, Lu J. Infrared spectroscopic analysis of 5,248 urinary stones from Chinese patients presenting with the first stone episode. Urol Res. 2011; 39:339–343. PMID: 21249491.

30. Najjar MF, Najjar F, Boukef K, Oueslati A, Memmi J, Bechraoui T. La lithiase infantile dans la région de Monastir étude clinique et biologique. Le Biologiste. 1986; 165:31–39.
31. Strohmaier WL, Weigl A. Jungers P, Daudon M, editors. Stone composition in upper Franconia-unusually high percentage of uric acid lithiasis. Renal stone disease. 1997. Amsterdam: Elsevier Science;p. 10–11.
32. Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997; 126:497–504. PMID: 9092314.

33. Grases F, Costa-Bauza A, Prieto RM. Renal lithiasis and nutrition. Nutr J. 2006; 5:23. PMID: 16956397.

34. Alaya A, Nouri A, Najjar MF. Urinary stone composition in pediatric patients: a retrospective study of 205 cases. Clin Chem Lab Med. 2011; 49:243–248. PMID: 21143010.

35. Agarwal BN, Cabebe FG. Renal acidification in elderly subjects. Nephron. 1980; 26:291–295. PMID: 7453916.

36. Daudon M, Jungers P. Diabetes and calculations. Feuillets de Biologie. 2001; 239:37–39.
37. Siener R, Glatz S, Nicolay C, Hesse A. The role of overweight and obesity in calcium oxalate stone formation. Obes Res. 2004; 12:106–113. PMID: 14742848.

38. Trinchieri A, Ostini F, Nespoli R, Rovera F, Montanari E, Zanetti G. A prospective study of recurrence rate and risk factors for recurrence after a first renal stone. J Urol. 1999; 162:27–30. PMID: 10379732.

39. Siener R, Glatz S, Nicolay C, Hesse A. Prospective study on the efficacy of a selective treatment and risk factors for relapse in recurrent calcium oxalate stone patients. Eur Urol. 2003; 44:467–474. PMID: 14499683.

40. Koşar A, Sarica K, Aydos K, Küpeli S, Türkölmez K, Göğüs O. Comparative study of long-term stone recurrence after extracorporeal shock wave lithotripsy and open stone surgery for kidney stones. Int J Urol. 1999; 6:125–129. PMID: 10226822.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download