Abstract
Background
We developed a single-color multitarget flow cytometry (SM-FC) assay, a single-tube assay with graded mean fluorescence intensities (MFIs). We evaluated the repeatability of SM-FC, and its correlation with multicolor flow cytometry (MFC), to assess its application as a routine FC assay.
Methods
We selected CD19, CD3, CD4, and CD8 as antigen targets to analyze a lymphocyte subset. MFIs were graded by adjusting monoclonal antibody (mAb) volumes to detect several cell populations. Dimly labeled mAb was prepared by decreasing mAb volume and the optimum diluted volume was determined by serial dilution. SM-FC repeatability was analyzed 10 times in 2 normal controls. The correlation between SM-FC and MFC was evaluated in 20 normal and 23 patient samples.
Results
CV values (0.8-5.0% and 1.3-4.1% in samples 1 and 2, respectively) acquired by SM-FC with CD3-fluorescein α-isothyocyanate (FITC)dim+CD4-FITCbright and with CD19-FITCdim+CD3-FITCbright showed good repeatability, comparable to that acquired by MFC (1.6-3.7% and 1.0-4.8% in samples 1 and 2, respectively). Excellent correlation was observed between the 2 methods in the 20 normal samples (B cells, T cells, non-Thelper cells, and Thelper cells; r2=0.87, 0.97, 0.97, and 0.98, respectively; P<0.05). There were also linear relationships between SM-FC with CD19-FITCdim+CD3-FITCbright and CD8-PEdim+CD4-PEbright, and MFC, in the 23 patient samples (B cells, T cells, Tcytotoxic cells, and Thelper cells; r2≥0.98, 0.99, 0.99, and 0.99, respectively; P<0.05).
Multicolor flow cytometry (MFC) is widely used in health research and treatment for a variety of tasks, such as providing the counts of helper-T lymphocytes needed to monitor the course and treatment of human immunodeficiency virus (HIV) infection [1-3], diagnosing and monitoring leukemia and lymphoma patients [4, 5], and evaluating peripheral blood hematopoietic stem cell grafts [6] and a variety of other diseases [7]. The technology is also used to cross-match organs for transplantation [8], and in research involving stem cells, apoptosis [9], phagocytosis [10], and a wide range of cellular properties including phenotype [11], cytokine expression [12], and cell-cycle status [13].
MFC can enumerate mature T, B, and natural killer (NK) cell populations, as well as CD4+and CD8+T-cell subsets, using 6 monoclonal antibodies (mAbs), including CD3, CD4, CD8, CD19, CD16, and CD56, in lymphocyte subset analyses [14-17]. Although some clinical laboratories routinely use a single-tube assay with lyse-no-wash methodology, which reduces inter-laboratory variability, a single-tube assay requires complex analysis with a multiple gating strategy [17-20]. The use of complex instruments with multicolor analysis, in which every fluorochrome has to be accurately compensated for, especially in a lyse-no-wash technique, can be problematic for an inexperienced operator [18].
With the goal of alleviating these difficulties, we have developed single-color multitarget flow cytometry (SM-FC), which circumvents the costly and labor-intensive procedures of manual preparation. The process is almost the same as MFC, except for the use of mAbs labeled with different mean fluorescence intensities (MFIs) of the same fluorochrome for detecting more than two cell populations, as a single-tube assay. We attempted to analyze a lymphocyte subset using this technique with graded MFIs by adjusting mAb volumes to detect several cell populations.
The aim of this study was to estimate the repeatability of SM-FC, evaluate the correlation between SM-FC and MFC, and assess the potential of the new technique as a routine flow cytometry (FC) approach. We selected CD19, CD3, CD4, and CD8 as antigen targets to demonstrate whether SM-FC is routinely applicable, because these antigens are expressed in a certain lymphocyte subset. Subset results obtained using SM-FC and MFC were compared in 23 patient samples.
To evaluate the repeatability of SM-FC and the correlation between SM-FC and MFC, we used 20 blood samples, obtained from adults who had visited our hospital for routine medical health check-ups. All individuals had displayed normal blood test results. Another 23 blood samples that had been obtained from patients for lymphocyte analysis were used to assess the potential of the novel technique as a routine FC approach. These patients had been variously diagnosed with aplastic anemia (N=4), myelodysplatic syndrome (N=3), AML (N=6), ALL (N=3), HIV infection (N=6), and infectious mononucleosis (N=1), but not initially with lymphoid malignancies such as ALL, CLL, and lymphoma. Sixteen patients with hematologic malignancies had a successful post-hematopoietic stem cell transplantation status for at least 6 months. Total white blood cell (WBC) count ranged from 1.33 to 14.54×109/L (median, 5.40×109/L). Lymphocyte count ranged from 0.49 to 6.12×109/L (median, 2.03×109/L). All blood samples were collected in vacutainer tubes coated with K2-EDTA (Becton-Dickinson, Franklin Lakes, NJ, USA) and were processed within 4 hr of blood collection.
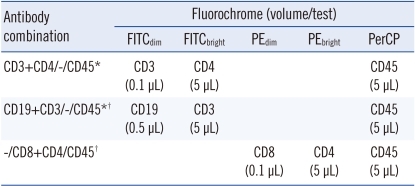
Six mAbs were used to evaluate the repeatability of SM-FC and the correlation between SM-FC and MFC. The mAbs were fluorescein α-isothyocyanate (FITC)-conjugated CD4, CD3, and CD19; phycoerythrin (PE)-conjugated CD8 and CD4; and peridinin chlorophyll protein complex (PerCP)-conjugated CD45 (BD Biosciences, San Jose, CA, USA). MFIs were graded by adjusting mAb volumes for detecting several cell populations (i.e., multitarget). Dimly labeled mAbs were prepared by decreasing mAb volume and the optimal diluted volume was determined using serial mAb dilutions. As a result, CD3 and CD19 FITC (0.1 and 0.5 µL/test, respectively) and CD8 PE (0.1 µL/test) yielded weakly positive cell populations in SM-FC. The mAb cocktails used in lymphocyte subset analysis are listed in Table 1. To compare with MFC, Multitest 6-color TBNK reagent (BD Biosciences), which contains 6 Abs, was used to identify and enumerate T, B, and NK lymphocyte subsets. A single-tube assay with the lyse-wash method was performed on 50 µL volumes of whole peripheral blood. A 6-color FACSCanto II flow cytometer (BD Biosciences) was used in accordance with the manufacturer's recommendation. Data were collected by adjusting the Fourier shell correlation (FSC) threshold to exclude platelets and debris. List mode data of 10,000 cells was collected according to predefined FSC threshold settings. Data analyses were conducted using the FACSDiva program (BD Biosciences).
The repeatability of SM-FC was analyzed 10 times in 2 normal controls. The correlation between SM-FC and MFC was evaluated in 20 normal samples. SM-FC with CD3-FITCdim+CD4-FITCbright was performed to detect dimly fluorescent non-Thelper cells and brightly fluorescent Thelper cells (Th cells). SM-FC with CD19-FITCdim+CD3-FITCbright was also achieved to detect weakly fluorescent B cells and strongly fluorescent T cells, respectively. To assess the potential of this method as a routine FC approach, SM-FC with CD19-FITCdim+CD3-FITCbright and CD8-PEdim+CD4-PEbright was performed to separate dimly fluorescent B cells, dimly fluorescent Tcytotoxic cells (Tc cells), and brightly fluorescent Th cells in 23 patient samples.
The correlation between cell populations obtained by SM-FC and MFC was assessed using the Pearson correlation coefficient and linear regression analysis. All statistical differences were considered significant if P<0.05. All statistical analyses were performed using MedCalc 12.0 software (MedCalc, Mariakerke, Belgium).
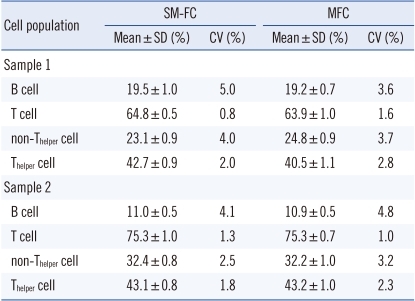
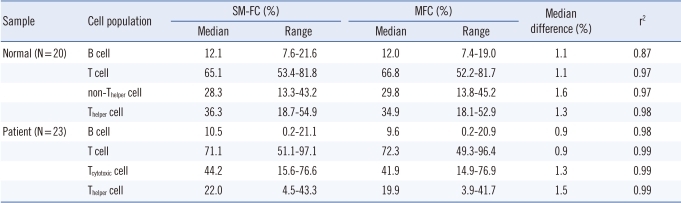
Negative, dimly fluorescent, and brightly fluorescent cell populations were clearly distinguished by SM-FC. SM-FC with CD3-FITCdim+CD4-FITCbright showed negative cells, dimly fluorescent non-Th cells, and brightly fluorescent Th cells. SM-FC with CD19-FITCdim+CD3-FITCbright presented negative cells, weakly fluorescent B cells, and strongly fluorescent T cells. The contours of the 3 peaks were sufficiently distinguishable and these markers could be readily set in each histogram (Fig. 1). Ten analyses in 2 normal controls revealed good repeatability based on the CV values (0.8-5.0% in sample 1; 1.3-4.1% in sample 2) acquired by SM-FC, comparable to those acquired by MFC (1.6-3.7% in sample 1; 1.0-4.8% in sample 2). The CVs of Th cells and non-Th cells obtained by SM-FC and MFC were lower than those of B cells (Table 2).
There was excellent correlation between SM-FC and MFC (P<0.05) in the 20 normal samples tested. Median differences between the 2 methods were <2%. SM-FC showed similar results to those of MFC, and agreed well with those obtained by MFC (Table 3).
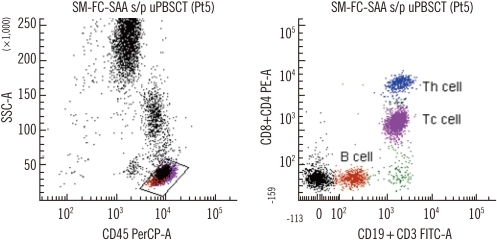
SM-FC adequately separated the lymphocyte population into 4 subpopulations (negative, dimly fluorescent B cells, dimly fluorescent Tc cells, and brightly fluorescent Th cells (Fig. 2). A linear relationship was evident between SM-FC and MFC in the 23 patient samples tested (P<0.05); the median difference between the 2 methods was <2% (Table 3).
The recent availability of MFC has allowed the absolute enumeration of the lymphocyte subpopulation in a single tube, including B lymphocytes or NK cells, at reduced cost and with shorter assay times [18-20]. Determination of lymphocyte subpopulations is very important in many kinds of immunological [20] and hematological disorders [21]. WBC differentiation based on FC recognition of cells has become a routine test [22-25]. However, since one fluorochrome is responsible for detecting only one expressed antigen, the resulting dot plot will only depict negative or positive cell populations in MFC. Because the number of available fluorochromes may be limited in a single tube, the resolution of MFC may be insufficient for the analysis of a large number of different cell populations in a single-tube assay format. To overcome the limited number of mAbs that can be used in a multicolor, single-tube technique, a sequential gating method has been attempted [17-20]. Although this approach permits enumeration of the lymphocyte subpopulation, the gating strategy is complex [17-20]. The separation of more than 2 cell populations using a single fluorochrome in the multicolor single-tube technique format exponentially increases the number of possible differential cell populations that can be enumerated.
The present results demonstrate the favorable repeatability of SM-FC and its correlation with MFC. Lymphocyte subsets including B cells, Th cells, and Tc cells were successfully verified using SM-FC with CD19-FITCdim+CD3-FITCbright and CD8-PEdim+CD4-PEbright in one dot plot, indicating the potential of SM-FC as a routine FC approach. Lymphocyte subpopulations were exclusively determined by antigenic expression; every included lymphocyte was positive for one or more markers and negative for others.
The most important contribution of SM-FC is that more antigen expression profiles can be obtained using multiple mAbs conjugated with the same fluorochromes at different intensities in a multicolor single-tube assay format. This approach is less sensitive to systematic error or bias because it allows for a larger lymphocyte window. This single-tube method approach produces the most complete determination of possible phenotypes, improving clinical interpretation and diagnosis. SM-FC also eliminates the need to control tube-to-tube population variability (quality control) with duplicate reagents, and excludes tube-to-tube window anomalies. Other practical advantages include decreased use of reagents and test tubes, which reduce labor-related costs. However, the routine use of diluted preparations of mAbs should be avoided, since subsaturation of a cell surface antigen could cause underexpression.
This novel technique has several limitations. First, most malignant cells such as leukemic blasts or lymphoma cells could present variable MFIs or aberrant expression due to their biological abnormalities [26]. The expression level of a particular target antigen may vary depending on the loss or gain of typical or aberrant antigen expression in hematological malignancies. This new method may be more useful to analyze cells that constantly express target surface antigens, for example, the lymphocyte subset in non-lymphoid malignancies, rather than to immunophenotype hematological malignancies. Secondly, some surface antigens, such as CD45, express different MFIs according to the type of normal leukocytes present [27]. Therefore, it may not be suitable to use mAbs that express various MFIs according to cell type in SM-FC. Finally, we have to carefully select the combination of dimly and brightly labeled mAbs in SM-FC, since it is not possible to distinguish a cell population stained with only brightly labeled mAbs from a population stained with dimly as well as brightly labeled mAbs using the same fluorochrome. It is better to combine mAbs like CD3 and CD19, which are not expressed in the same cell population, using the same fluorochrome with different intensities. Antigen coexpression may lead to overlap between dimly and brightly labeled mAbs, and cell populations would not be clearly distinguishable, leading to inaccurate results.
In conclusion, SM-FC not only displays acceptable repeatability and correlation in normal samples, but also has potential as a routine FC technique. Because there are some difficulties to apply to all clinical areas, this method should be applied to analyze cell populations that present constant levels of antigens in non-hematological malignancies or other diseases that do not influence the level of antigen expression. In spite of several current limitations, SM-FC could be a potential alternative tool, as a multicolor, single-tube technique, for the routine clinical laboratory detection of a lymphocyte subset.
References
1. Hengel RL, Nicholson JK. An update on the use of flow cytometry in HIV infection and AIDS. Clin Lab Med. 2001; 21:841–856. PMID: 11770291.
2. Illoh OC. Current applications of flow cytometry in the diagnosis of primary immunodeficiency diseases. Arch Pathol Lab Med. 2004; 128:23–31. PMID: 14692816.

3. Mandy FF. Twenty-five years of clinical flow cytometry: AIDS accelerated global instrument distribution. Cytometry A. 2004; 58:55–56. PMID: 14994221.

4. Braylan RC. Impact of flow cytometry on the diagnosis and characterization of lymphomas, chronic lymphoproliferative disorders and plasma cell neoplasias. Cytometry A. 2004; 58:57–61. PMID: 14994222.

5. Orfao A, Ortuño F, de Santiago M, Lopez A, San Miguel J. Immunophenotyping of acute leukemias and myelodysplastic syndromes. Cytometry A. 2004; 58:62–71. PMID: 14994223.

6. Keeney M, Gratama JW, Sutherland DR. Critical role of flow cytometry in evaluating peripheral blood hematopoietic stem cell grafts. Cytometry A. 2004; 58:72–75. PMID: 14994224.

7. Bagwell CB. DNA histogram analysis for node-negative breast cancer. Cytometry A. 2004; 58:76–78. PMID: 14994225.

8. Maecker HT, Maino VC, editors. Manual of Clinical Laboratory Immunology. 2002. 6th ed. Washington, DC: ASM Press;p. 338–346.
9. Vermes I, Haanen C, Reutelingsperger C. Flow cytometry of apoptotic cell death. J Immunol Methods. 2000; 243:167–190. PMID: 10986414.

10. Lehmann AK, Sornes S, Halstensen A. Phagocytosis: measurement by flow cytometry. J Immunol Methods. 2000; 243:229–242. PMID: 10986417.

11. Krutzik PO, Irish JM, Nolan GP, Perez OD. Analysis of protein phosphorylation and cellular signaling events by flow cytometry: techniques and clinical applications. Clin Immunol. 2004; 110:206–221. PMID: 15047199.

12. Pala P, Hussell T, Openshaw PJ. Flow cytometric measurement of intracellular cytokines. J Immunol Methods. 2000; 243:107–124. PMID: 10986410.

13. Pozarowski P, Darzynkiewicz Z. Analysis of cell cycle by flow cytometry. Methods Mol Biol. 2004; 281:301–311. PMID: 15220539.

14. Gratama JW, Kraan J, Keeney M, Granger V, Barnett D. Reduction of variation in T-cell subset enumeration among 55 laboratories using single-platform, three or four-color flow cytometry based on CD45 and SSC-based gating of lymphocytes. Cytometry. 2002; 50:92–101. PMID: 12116351.

15. Alamo AL, Melnick SJ. Clinical application of four and five-color flow cytometry lymphocyte subset immunophenotyping. Cytometry. 2000; 42:363–370. PMID: 11135290.

16. Chng WJ, Tan GB, Kuperan P. Establishment of adult peripheral blood lymphocyte subset reference range for an Asian population by single-platform flow cytometry: influence of age, sex, and race and comparison with other published studies. Clin Diagn Lab Immunol. 2004; 11:168–173. PMID: 14715565.

17. Autissier P, Soulas C, Burdo TH, Williams KC. Evaluation of a 12-color flow cytometry panel to study lymphocyte, monocyte, and dendritic cell subsets in humans. Cytometry A. 2010; 77:410–419. PMID: 20099249.

18. Colombo F, Cattaneo A, Lopa R, Portararo P, Rebulla P, Porretti L. Evaluation of a multicolor, single-tube technique to enumerate lymphocyte subpopulations. Clin Vaccine Immunol. 2008; 15:1124–1127. PMID: 18448621.

19. Lambert C, Cristina I, Christian G. Enumeration of peripheral lymphocyte subsets using 6 vs. 4 color staining: a clinical evaluation of a new flowcytometer. Cytometry B Clin Cytom. 2006; 70:29–38. PMID: 16353133.

20. Ashman M, Sachdeva N, Davila L, Scott G, Mitchell C, Cintron L, et al. Influence of 4- and 6-color flow cytometers and acquisition/analysis softwares on the determination of lymphocyte subsets in HIV infection. Cytometry B Clin Cytom. 2007; 72:380–386. PMID: 17226862.

21. Szczepański T, van der Velden VH, van Dongen JJ. Flow-cytometric immunophenotyping of normal and malignant lymphocytes. Clin Chem Lab Med. 2006; 44:775–796. PMID: 16776621.

22. Roussel M, Benard C, Ly-Sunnaram B, Fest T. Refining the white blood cell differential: the first flow cytometry routine application. Cytometry A. 2010; 77:552–563. PMID: 20506466.

23. Cherian S, Levin G, Lo WY, Mauck M, Kuhn D, Lee C, et al. Evaluation of an 8-color flow cytometric reference method for white blood cell differential enumeration. Cytometry B Clin Cytom. 2010; 78:319–328. PMID: 20533390.

24. Björnsson S, Wahlström S, Norström E, Bernevi I, O'Neill U, Johansson E, et al. Total nucleated cell differential for blood and bone marrow using a single tube in a five-color flow cytometer. Cytometry B Clin Cytom. 2008; 74:91–103. PMID: 18061952.

25. Faucher JL, Lacronique-Gazaille C, Frébet E, Trimoreau F, Donnard M, Bordessoule D, et al. "6 markers/5 colors" extended white blood cell differential by flow cytometry. Cytometry A. 2007; 71:934–944. PMID: 17879238.

26. Swerdlow SH, Campo E, editors. WHO classification tumours of haematopoietic and lymphoid tissues. 2008. 4th ed. Lyon: IARC press;p. 109–320.
27. Nicholson JK, Hubbard M, Jones BM. Use of CD45 fluorescence and side-scatter characteristics for gating lymphocytes when using the whole blood lysis procedure and flow cytometry. Cytometry. 1996; 26:16–21. PMID: 8809476.

Fig. 1
Representative dot plots and histograms of single-color multitarget flow cytometry with CD19-FITCdim+CD3-FITCbright and with CD3-FITCdim+CD4-FITCbright. (A) B cells (CD19-FITCdim, brown) and T cells (CD3-FITCbright, green). (B) Non-Thelper cells (CD3-FITCdim, pink) and Thelper cells (CD3-FITCdim+CD4-FTTCbright, blue).
Abbreviations: SM-FC, single-color multitarget flow cytometry; PerCP, peridinin chlorophyll protein complex; FITC, fluorescein α-isothyocyanate.
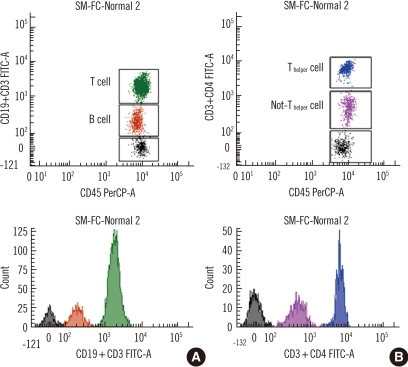
Fig. 2
Representative dot plots of single-color multitarget flow cytometry with CD19-FITCdim+CD3-FITCbright and CD8-PEdim+CD4-PEbright. B cells (CD19-FITCdim, brown), Tcytotoxic cells (CD3-FITCbright+CD8-PEdim, pink), and Thelper cells (CD3-FITCbright+CD4-PEbright, blue) in a right dot plot.
Abbreviations: SM-FC-SAA s/p uPBSCT, single-color multitarget flow cytometry-severe aplastic anemia after unrelated peripheral blood stem cell transplantation; SSC-A, side scatter; PerCP, peridinin chlorophyll protein complex; PE, phycoerythrin; FITC, fluorescein α-isothyocyanate.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download