Abstract
B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma (BL) (intermediate DLBCL/BL), is a heterogeneous group with some features resembling DLBCL and others resembling BL. Here, we report a case of intermediate DLBCL/BL in a Korean child. A 2-yr-old male was admitted for evaluation and management of left hip pain. Immunohistochemistry of a biopsy of the femur neck revealed tumor cells positive for CD20, CD10, BCL2, BCL6, and Ki67. A bone marrow (BM) aspirate smear revealed that 49.3% of all nucleated cells were abnormal lymphoid cells, composed of large- and medium-sized cells. Immunophenotyping of the neoplastic cells revealed positivity for CD19, CD10, CD20, and sIg lambda and negativity for CD34, Tdt, and myeloperoxidase (MPO). Cytogenetic and FISH analyses showed a complex karyotype, including t(8;14)(q24.1;q32) and IGH-MYC fusion. Intensive chemotherapy was initiated, including prednisone, vincristine, L-asparaginase, daunorubicin, and central nervous system prophylaxis with intrathecal methotrexate (MTX) and cytarabine. One month after the initial diagnosis, BM examination revealed the persistent of abnormal lymphoid cells; cerebrospinal fluid cytology, including cytospin, showed atypical lymphoid cells. The patient was treated again with cyclophosphamide, vincristine, prednisone, adriamycin, MTX, and intrathecal MTX and cytarabine. The patient died of sepsis 5 months after the second round of chemotherapy.
In most cases, a better understanding of the molecular pathogenesis underlying lymphoid malignancies has allowed for a pathogenesis-based approach to lymphoma classification [1, 2]. Improved immunophenotyping and genetic studies have increased the recognition of lymphoma cases that lie on a biologic continuum between 2 entities [3]. Specifically, these borderline categories, known as "gray zone lymphoma," "atypical Burkitt lymphoma (BL)," or "Burkitt-like lymphoma (BLL)," have overlapping clinical, morphological, and/or immunophenotypic features belonging to diffuse large B-cell lymphoma (DLBCL) and BL. The 2008 WHO classification includes provisional borderline categories for cases that are not clearly DLBCL or BL. This new category is called as B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and BL (intermediate DLBCL/BL) [4]. Many cases of intermediate DLBCL/BL that morphologically resemble BL occur in adults and are rare in patients under the age of 18 [5]. In general, these cases have a poor prognosis, with the exception of children. In children, intermediate DLBCL/BL does not have an adverse prognosis, based on gene expression profiling (GEP), although the number of patients studied is low. The prognosis for young adults is still unclear [6]. Herein, we report a case of intermediate DLBCL/BL in a Korean child and review the relevant literature.
A 2-yr-old male was admitted for evaluation and management of left hip pain. On admission, physical examination was significant for several palpable lymph nodes in the left inguinal area and an ill-defined heterogeneous mass-like lesion in the left anteromedial muscle. Abdominopelvic computed tomography and magnetic resonance imaging of both hips were performed on suspicion of myositis. Radiologic studies suggested osteomyelitis of the left proximal femur with subperiosteal abscess, myositis, and a small-cell tumor, such as lymphoma, leukemia, or Ewing's sarcoma. Bone biopsy of the femur neck, bone marrow (BM) aspiration, and biopsy of both posterior superior iliac crests were performed. Immunohistochemistry on the biopsy specimen of the femur neck revealed tumor cells positive for CD20, CD10, BCL2, BCL6, CD99, and Ki67 (-90%) and negative for myeloperoxidase (MPO), Tdt, CD3, and cyclin D1 (Fig. 1). In situ hybridization for Epstein-Barr virus-encoded RNAs (EBER) was negative in the tumor cells. The final diagnosis from the bone biopsy was high-grade B-cell lymphoma, suggestive of B lymphoblastic lymphoma. Peripheral blood examination revealed the following: hemoglobin, 9.6 g/dL; white blood cell count, 6.83×109/L; platelet count, 182×109/L, and 5 atypical lymphocytes per 100 white blood cells. Based on the laboratory findings and bone biopsy results, we suspected lymphoma or lymphoblastic leukemia; therefore, we performed BM examination, cytogenetic analysis, and immunophenotyping. The BM biopsy was insufficient for evaluating cellularity; 49.3% of all nucleated cells were abnormal lymphoid cells, consisting of large- and medium-sized cells. Large neoplastic cells had irregular nuclei with 1-2 distinct nucleoli and abundant deeply basophilic cytoplasm. Medium-sized cells had round nuclei with 1-4 prominent nucleoli and scantly to moderately basophilic cytoplasm with some vacuoles (Fig. 2). Immunophenotyping of the neoplastic cells revealed positivity for CD45 (99.0%), CD19 (94.84%), CD10 (27.64%), CD20 (94.24%), HLA-DR (95.70%), sIg lambda (96.45%), CD13 (22.46%), and CD117 (22.22%) and negativity for CD34 (0.01%), Tdt (0.64%), MPO (0.01%), CD33 (1.58%), CD14 (1.18%), CD41 (3.08%), CD2 (3.00%), sCD3 (7.78%), CD5 (1.42%), CD7 (1.34%), and CD56 (5.82%). Cytogenetic analysis of the cells in the BM aspirates revealed that the cells had the following karyotypes: 46,XY, t(8;14)(q24.1;q32),del(11)(q13),dup(11)(q22q13),der(17)del(17)(p12)t(1;17)(q21;q25)[29]/46,idem,t(12;19)(q13;p13.2)[4]/46,idem,add(19))(p11)[4]/46,idem,add(13)(q34)[3] (Fig. 3). FISH analysis of BM aspirate cells was performed using Vysis LSI IGH/MYC, CEP 8 tri-color, dual fusion translocation probes (Abbott Molecular, Des Plaines, IL, USA). We detected an IGH-MYC rearrangement in 81.2% of the nuclei examined with typical 2 fusions, 1 orange, 1 green, and 2 aqua signals, which was described as nuc ish (D8Z2×2,MYC×3,IGH×3)(MYC con IGH×2)[325/400] (Fig. 4). Neither BCL2 nor BCL6 rearrangements were observed by FISH analysis on BM aspirates cells using Vysis LSI BCL2 and BCL6 dual color, break apart rearrangement probes (Abbott Molecular). The patient was diagnosed with intermediate DLBCL/BL. Intensive chemotherapy with prednisone, vincristine, L-asparaginase, daunorubicin, and central nervous system prophylaxis with intrathecal methotrexate (MTX) and cytarabine were initiated. One month after the initial diagnosis, follow-up BM examination demonstrated persistence of abnormal lymphoid cells. Cerebrospinal fluid (CSF) analysis was performed after induction of chemotherapy; CSF cytology, including cytospin, showed atypical lymphoid cells consistent with malignant lymphoma. The patient was treated again with cyclophosphamide, vincristine, prednisone, adriamycin, MTX, and intrathecal MTX and cytarabine. The patient died of sepsis 5 months after initiation of the second round of chemotherapy.
Gray zone B-cell lymphoma, such as intermediate DLBCL/BL, cannot be classified into a single distinct disease entity. This new category of lymphoma has morphologic, immunophenotypic, and genetic features that include aspects of both DLBCL and BL, but differ with respect to one or more findings [7]. The 2008 WHO classification affirms that the following lymphoma cases should not be diagnosed as intermediate DLBCL/BL: those with a typical DLBCL morphology and a very high proliferation index, typical DLBCL with a MYC translocation, typical BL without a MYC rearrangement, and those with IG-MYC rearrangement as the only abnormality [4].
Intermediate DLBCL/BL most often occurs in adults, some with a history of follicular lymphoma; it is extremely rare in pediatric patients. The majority of patients present with generalized lymphadenopathy or mass lesions in extranodal sites and frequent involvement of the BM. Some patients have a leukemic presentation [5, 8]. Liang et al. [8] reported the clinicopathologic features of 2 pediatric patients with gray zone lymphoma, who presented with common features, such as male gender, older than 10 yr of age at the time of diagnosis, and presentation with a mediastinal mass. In children, high cure rates are achieved with treatment strategies similar or identical to those for BL and DLBCL [9]. The gray zone between BL and DLBCL currently does not impact therapy decision or outcome in childhood lymphomas [7].
Morphologic feature are useful in the differential diagnosis of intermediate DLBCL/BL. Such morphologic features include variable cellular forms, such as those smaller than typical DLBCL, those resembling BL cells, those larger than typical BL, and those resembling DLBCL cells. The immunophenotype is similar to BL, showing positivity for CD19, CD20, CD22, CD79a, and germinal center-associated molecules, CD10 and BCL6. BCL2 expression may be absent, weak, or strong, and the Ki67 labeling index shows varying positivity [3]. Genetically, 35-50% of cases of intermediate DLBCL/BL show 8q24/MYC translocations but often with atypical features, including one or more of the following: (1) rearrangement with a non-IG partner, (2) part of a complex karyotype, and (3) concurrent rearrangements of the BCL2 and/or BCL6 genes, suggesting a "double-hit" or "triple-hit" lymphoma. Double-hit lymphomas are characterized by a second translocation in addition to t(8;14), t(8;22), or t(2;8). In the majority of the double-hit cases, an 18q21/BCL2 breakpoint can be found, mostly as a t(8;14) plus t(14;18) karyotype [10-13]. Double-hit lymphomas are almost always absent in children, consistent with the almost complete lack of t(14;18) found among lymphomas in those younger than 18 yr [6].
More recently, GEP analysis using microarrays can establish molecular categories within the gray zone between DLBCL and BL [1]. The bioinformatic core group extension method applied by the Molecular Mechanisms in Malignant Lymphomas group identified a set of 53 mature aggressive B-cell lymphomas with a molecular BL (mBL) index between that of mBL (mBL index >0.95) and that of non-mBL (mBL index <0.05). These lymphomas could not be classified as mBL or as non-mBL and were called "molecular intermediate lymphomas" [1, 5, 6]. Molecular intermediate DLBCL/BL in children has a significantly higher Burkitt index by GEP than in adult patients, frequent IG-MYC positivity, and a good outcome. Interestingly, significant numbers (31%) of morphologic DLBCL in children show mBL by GEP, with more than half of them having IG-MYC. These findings suggest that, in children, biologic BL might be hidden among DLBCL [6]. Salaverria et al. [6] suggested that GEP should be considered a single diagnostic criterion, like MYC status or CD10 positivity.
In the present case, the patient was diagnosed with intermediate DLBCL/BL based on the intermediate morphological features of both BL and DLBCL, the expression of CD10, BCL6, BCL2, and Ki67 labeling index, and a complex karyotype with 8q34/MYC.
Based on existing literature and the findings of this case, evaluation for intermediate DLBCL/BL should include an immunophenotypic panel with CD10, BCL6, BCL2, and Ki67. In addition, evaluation should include conventional cytogenetic analysis for detection of simple or complex karyotypic abnormalities and molecular cytogenetic evaluation (FISH) with MYC-IgH fusion probe for t(8;14), BCL2-IgH fusion probe for t(14;18), BCL2 break-apart probe, BCL6 break-apart probe, MYC break-apart probe, IGH break-apart probe, and IGL break-apart probe for t(2;8) and t(8;22) [12].
However, several questions remain unanswered. A major concern is that cases of intermediate DLBCL/BL only slightly differ from BL and DLBCL. Therefore, it is important that the diagnostic border of DLBCL and BL is clearly defined in order to identify the specific morphology, immunophenotyping, genetics, and molecular lesions based on a gene expression. Another issue is that the current treatment of intermediate DLBCL/BL may compromise the validity of clinical trials evaluating the efficacy and safety of therapies on well-established diagnostic entities and may obscure the pathologic predictors of their outcome and treatment response.
Figures and Tables
 | Fig. 1Immunohistochemistry shows positivity for BCL2 (A), BCL6 (B), CD10 (C), and Ki67 (D) of tumor cells (Femur neck, immunohistochemical stains, 400× magnification). |
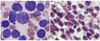 | Fig. 2Bone marrow smear (A, Wright-Giemsa stain, 1,000× magnification) and biopsy (B, H&E stain, 1,000× magnification) reveal abnormal lymphoid cells composed of large and medium sized cells. Large neoplastic cells showed irregular nuclei with 1-2 distinct nucleoli and abundant deeply basophilic cytoplasm. Medium-sized cells showed round nuclei with 1-4 prominent nucleoli and had scantly to moderately basophilic cytoplasm with some vacuoles. |
References
1. Hummel M, Bentink S, Berger H, Klapper W, Wessendorf S, Barth TF, et al. A biologic definition of Burkitt's lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006. 354:2419–2430.

2. Saglio G, Bosa M, Gino M, Ulisciani S, Parvis G. Molecular pathogenesis of diffuse large B-cell lymphoma. Hematologica reports. 2006. 2:68–69.
3. Carbone A, Gloghini A, Aiello A, Testi A, Cabras A. B-cell lymphomas with features intermediate between distinct pathologic entities. From pathogenesis to pathology. Hum Pathol. 2010. 41:621–631.

4. Kluin PM, Harris NL, Stein H, Leoncini L, Raphael M, Campo E, et al. Swerdlow SH, Campo E, editors. B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma. WHO classification of tumours of haematopoietic and lymphoid tissues. 2008. 4th ed. Lyon: IARC Press;265–266.
5. Klapper W, Szczepanowski M, Burkhardt B, Berger H, Rosolowski M, Bentink S, et al. Molecular profiling of pediatric mature B-cell lymphoma treated in population-based prospective clinical trials. Blood. 2008. 112:1374–1381.

6. Salaverria I, Siebert R. The gray zone between Burkitt's lymphoma and diffuse large B-cell lymphoma from a genetics perspective. J Clin Oncol. 2011. 29:1835–1843.

7. Hasserjian RP, Ott G, Elenitoba-Johnson KS, Balague-Ponz O, de Jong D, de Leval L. Commentary on the WHO classification of tumors of lymphoid tissues (2008): "Gray zone" lymphomas overlapping with Burkitt lymphoma or classical Hodgkin lymphoma. J Hematop. 2009. 2:89–95.

8. Liang X, Greffe B, Cook B, Giller R, Graham DK, McGranahan AN, et al. Gray zone lymphomas in pediatric patients. Pediatr Dev Pathol. 2011. 14:57–63.

9. Patte C, Auperin A, Gerrard M, Michon J, Pinkerton R, Sposto R, et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. 2007. 109:2773–2780.

10. Quintanilla-Martinez L, de Jong D, de Mascarel A, Hsi ED, Kluin P, Natkunam Y, et al. Gray zones around diffuse large B cell lymphoma. Conclusions based on the workshop of the XIV meeting of the European Association for Hematopathology and the Society of Hematopathology in Bordeaux, France. J Hematop. 2009. 2:211–236.

11. Le Gouill S, Talmant P, Touzeau C, Moreau A, Garand R, Juge-Morineau N, et al. The clinical presentation and prognosis of diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC rearrangement. Haematologica. 2007. 92:1335–1342.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download