Abstract
Background
Recent studies and case reports have shown that recombinant factor VIIa (rFVIIa) treatment is effective for reversing coagulopathy and reducing blood transfusion requirements in trauma patients with life-threatening hemorrhage. The purpose of this study is to evaluate the effect of rFVIIa treatment on clinical outcomes and cost effectiveness in trauma patients.
Methods
Between January 2007 and December 2010, we reviewed the medical records of patients who were treated with rFVIIa (N=18) or without rFVIIa (N=36) for life-threatening hemorrhage due to multiple traumas at the Emergency Department of Pusan National University Hospital in Busan, Korea. We reviewed patient demographics, baseline characteristics, initial vital signs, laboratory test results, and number of units transfused, and then analyzed clinical outcomes and 24-hr and 30-day mortality rates. Thromboembolic events were monitored in all patients. Transfusion costs and hospital stay costs were also calculated.
Results
In the rFVIIa-treated group, laboratory test results and clinical outcomes improved, and the 24-hr mortality rate decreased compared to that in the untreated group; however, 30-day mortality rate did not differ between the groups. Thromboembolic events did not occur in both groups. Transfusion and hospital stay costs in the rFVIIa-treated group were cost effective; however, total treatment costs, including the cost of rFVIIa, were not cost effective.
Massive blood transfusion is inevitable for multiple trauma patients with life-threatening hemorrhage. However, numerous complications of massive blood transfusion, such as dilutional thrombocytopenia, coagulation factor deficiency, disseminated intravascular coagulation, hypothermia, acidosis, citrate intoxication, hyperkalemia, transfusion reactions, and infections can occur [1, 2]. In recent years, there have been many studies focused on reducing the transfusion requirements in multiple trauma patients to decrease the risks and complications associated with massive blood transfusion.
Recombinant activated factor VII (rFVIIa; NovoSeven; Novo Nordisk Pharmaceuticals, Bagsvaerd, Denmark), a trypsin-like serine protease that plays a key role in the blood coagulation cascade [3], has also been used to decrease blood transfusion requirements in patients with multiple trauma and life-threatening hemorrhage. Initially, rFVIIa was used in patients with congenital or acquired hemophilia along with inhibiting antibodies against factor VIII or IX. Currently, rFVIIa is licensed for use in hemophiliacs in the USA, Europe, and many other countries throughout the world [4].
In the last few years, rFVIIa has also been used as a universal hemostatic agent for the following conditions:congenital factor VII deficiencies, platelet disorders, other coagulation factor defects, liver disorders, liver transplantation, surgery, trauma, and reversal of anti-coagulation therapy [5]. Kenet et al. reported the first successful use of rFVIIa in a 19-yr-old soldier with traumatic coagulopathy associated with a rifle injury [6]. Since that time, several studies and case reports have shown that the use of rFVIIa is effective in reversing coagulopathy and reducing blood transfusion requirements in trauma patients with life-threatening hemorrhage.
However, the limited number of studies has not determined whether rFVIIa treatment in trauma patients improves clinical outcome, reduces mortality, and is cost effective. Therefore, this issue is still controversial. Here, we conducted a retrospective cohort study of multiple trauma patients with life-threatening hemorrhage who received massive transfusions to evaluate the effect of rFVIIa treatment on clinical outcomes, mortality rate, and cost effectiveness by comparing rFVIIa-treated patients to patients not treated with rFVIIa.
We reviewed the medical records of all trauma patients who were treated in the Emergency Department of Pusan National University Hospital in Busan, Korea between January 2007 and December 2010 in a retrospective cohort study. This study was approved by the Institutional Review Board of our hospital. We selected multiple trauma patients with life-threatening hemorrhage who were injured in a traffic accident, falling accident, or crushing accident, and received ≥8 units of packed red blood cells (pRBCs) within the first 24 hr of hospitalization. We excluded patients with hemophilia or other coagulation disorders as well as pediatric and adolescent patients <18 yr of age.
The use of rFVIIa was indicated by the following 2 criteria: massive hemorrhage due to multiple trauma resulting in hemorrhagic shock with an initial systolic blood pressure <90 mmHg, and increased transfusion requirements due to continuous, life-threatening hemorrhage. In the Emergency Department of Pusan National University Hospital, rFVIIa has been approved for use by emergency medicine specialists. Patients who received the rFVIIa drug were injected with a single 240 KIU (a 4.8 mg vial) dose intravenously after informed consent on the necessity, adverse effects, and high cost of the rFVIIa drug. For non-approved use of rFVIIa, a 240 KIU dose (a 4.8 mg vial) is administered to an adult patient weighing 50-100 kg to achieve a 50-100 µg/kg dose as recommended according to the study by Goodnough and Shander [7].
We also selected patients who were not treated with rFVIIa from the multiple trauma patients with life-threatening hemorrhage who were injured by a traffic accident, falling accident, or crushing accident, and received ≥8 units of pRBCs within the first 24 hr of hospitalization. The rFVIIa-untreated patients also had massive hemorrhage due to multiple trauma resulting in hemorrhagic shock with an initial systolic blood pressure <90 mmHg and increased transfusion requirements due to continuous life-threatening hemorrhage. However, they refused the use of rFVIIa because of the extremely high price of the rFVIIa drug. We recorded the time of admission and the time of rFVIIa administration to determine the corresponding time interval. We eliminated from the analysis those patients who received rFVIIa after the first 24 hr of admission. A total of 18 patients who were treated with rFVIIa and 36 patients who were not treated with rFVIIa were selected for this study. In addition, 12 patients from the study by Joo et al. [8] were also included in the rFVIIa-treated group in our study.
We obtained and reviewed the following information from all selected patients :gender, age, weight, mechanism of injury, bleeding site, injury severity score (ISS), and abbreviated injury scale score (AIS). We obtained initial data of body temperature, systolic blood pressure, arterial pH, base deficit, and lactic acid level, as well as the changes in hemoglobin level, platelet count, prothrombin time (PT), and activated partial thromboplastin time (aPTT) for all patients.
The units of pRBCs, fresh frozen plasma (FFP), and platelet (PLT) concentrate transfused before and after rFVIIa administration within the first 24 hr of hospitalization were recorded for the rFVIIa-treated patients. We also reviewed the total units of pRBCs, FFP, and PLT concentrate transfused during the first 24 hr of hospitalization for both the rFVIIa-treated and rFVIIa-untreated patients.
We analyzed the clinical outcomes, including the duration of intensive care unit (ICU) stay, the duration of hospital stay, and the mortality rate in the first 24 hr and 30 days. The characteristics of the rFVIIa-treated patients who were alive or dead at 30 days after admission were compared and analyzed. The transfusion costs and hospital stay costs for both the rFVIIa-treated and rFVIIa-untreated patients were calculated. The transfusion costs were calculated by multiplying the cost of pRBCs, FFP, and PLT concentrates per unit by the number of units used. The hospital stay costs were calculated by multiplying the cost of one admission day at our hospital by the total number of admission days. Total treatment cost was the sum total of the transfusion costs, the hospital stay costs, and the price of the rFVIIa drug.
Administration of rFVIIa was the independent variable. Other covariates analyzed included gender, age, mechanism of injury, ISS, AIS (head, face, chest, abdomen, extremities, and external), initial systolic blood pressure, body temperature, arterial pH, base deficit, lactic acid level, hemoglobin level, platelet count, PT, aPTT, number of units transfused, ICU stay, hospital stay, and mortality rate. All data were analyzed using MedCalc software (version 11.5; MedCalc Software, Mariakerke, Belgium). Descriptive statistics for variables were expressed as the mean±SD. The Mann-Whitney U test was used to compare variables between the rFVIIa-treated and rFVIIa-untreated patients. A P value of <0.05 was considered statistically significant.
A total of 214 patients who sustained multiple trauma were treated in the Emergency Department of Pusan National University Hospital between January 2007 and December 2010. Among them, 70 patients received ≥8 units of pRBCs within the first 24 hr of hospitalization. After patients were eliminated by the exclusion criteria described above, 18 patients who were treated with rFVIIa and 36 patients who were not treated with rFVIIa were selected for this study. All rFVIIa-treated patients were intravenously injected with 240 KIU (a 4.8 mg vial) of rFVIIa. The time interval from hospital admission to rFVIIa administration was an average of 3.3 (±2.8) hr.
Demographics and baseline characteristics of rFVIIa-treated and rFVIIa-untreated patients are shown in Table 1. The male: female ratio was 2:1 in the 18 rFVIIa-treated, and 3:1 in the 36 rFVIIa-untreated patients. The mean age of the rFVIIa-treated and rFVIIa-untreated patients was 45.9 yr (range, 26-80 yr) and 48.7 yr (range, 20-79 yr), respectively (P =0.5509). Of the rFVIIa-treated patients, 9 (50%), 8 (44%), and 1 (6%) sustained trauma from traffic accidents, falls, and crushing accidents, respectively, and 20 (56%), 14 (39%), and 2 (6%) of the rFVIIa-untreated patients sustained trauma from traffic accidents, falls, and crushing accidents, respectively.
Transfusion units before and after rFVIIa administration within the first 24 hr of admission in the 18 rFVIIa-treated patients are presented in Fig. 1. The number of units transfused within the first 24 hr decreased significantly after rFVIIa administration, and before vs. after rFVIIa administration, the number of unit (mean±SD) was as follows:pRBCs 11.1±3.9 vs. 3.2±2.8, P <0.0001; FFP 9.8±5.1 vs. 2.9±2.4, P <0.0001; and PLT concentrate 6.4±6.8 vs. 1.2±2.4, P =0.0085. The average time interval from hospital admission to rFVIIa administration in the rFVIIa-treated patients was 3.3 hr. In contrast, the number of transfusion units used in the 36 rFVIIa-untreated patients within the first 24 hr before vs. after 3.3 hr of admission did not differ significantly; pRBCs 9.4±3.6 vs. 10.2±4.9, P =0.4115; FFP 6.7±3.0 vs. 8.3±5.2, P =0.1208; and PLT concentrate 4.9±4.3 vs. 6.6±6.7, P=0.2052.
The number of transfusion units used within the first 24 hr of admission and during the entire hospital stay is shown in Table 2. The data revealed that rFVIIa-treated patients required fewer units of pRBCs and FFP than rFVIIa-untreated patients. The number of PLT concentrate units transfused did not differ significantly between the 2 groups in the independent samples t test. However, the number of PLT concentrate units transfused was significantly reduced in a multiple regression analysis (P < 0.001).
Changes in the mean value of hemoglobin, platelet count, PT, and aPTT after rFVIIa administration in the rFVIIa-treated group and those within the first 24 hr of admission in the rFVIIa-untreated group are shown Fig. 2. Although hemoglobin levels in the rFVIIa-treated group increased until 3 hr after rFVIIa administration, those in the rFVIIa-untreated group decreased. Platelet counts decreased in both groups after 24 hr of admission; however, the platelet count decrease in the rFVIIa-treated group was less prominent than that in the rFVIIa-untreated group. The PT and aPTT values of the rFVIIa-untreated group were prolonged for the first 3 hr, whereas the PT and aPTT values of the rFVIIa-treated group decreased to the normal range until 6 hr after rFVIIa administration.
Clinical outcomes are shown in Table 3. In rFVIIa-treated group, 1 patient died within 24 hr due to hypovolemic shock, while 3 patients died due to hypovolemic shock and 1 from multiple organ failure within 30 days. In the rFVIIa-untreated group, 2 patients died due to hypovolemic shock, 1 from multiple organ failure, and 1 from severe lung contusion within 24 hr, while 3 patients died due to hypovolemic shock, 2 from septic shock, and 1 from chronic renal failure within 30 days.
Known fatal complications of the rFVIIa drug, including thromboembolic events such as acute myocardial infarction or cerebrovascular infarction, did not occur in the rFVIIa-treated group. Thromboembolic events also did not occur in the rFVIIa-untreated group.
Transfusion costs in the rFVIIa-treated and rFVIIa-untreated groups were 2,174,217 won and 3,125,246 won, respectively (P =0.0084); total hospital stay costs were 2,218,275 won and 5,478,900 won, respectively (P =0.0017); and total treatment costs, including the cost of the rFVIIa drug, were 8,807,692 won and 8,604,146 won, respectively (P =0.5571). Therefore, rFVIIa treatment could reduce transfusion costs and hospital stay costs; however, the total treatment cost, including the price of rFVIIa drug, was not cost effective.
We compared the characteristics of the rFVIIa-treated patients who were alive 30 days after admission to those of the rFVIIa-treated patients who were deceased (Table 4). As shown in Table 4, older age, lower initial systolic blood pressure, more acidic arterial pH, higher lactic acid level, prolonged PT and aPTT values, and massive transfusion of pRBCs and FFP were statistically significant prognostic factors for worse survival in multiple trauma patients with life-threatening hemorrhage.
Since the first report on the successful use of rFVIIa by Kenet et al. [6], many studies have reported on the use of rFVIIa in patients with profuse bleeding due to severe trauma. Martinowitz et al. reported that the use of rFVIIa led to cessation of diffuse bleeding and decreased the blood transfusion requirement to 2 units of pRBCs with reduced PT and aPTT in 7 multiple-transfused trauma patients with life-threatening hemorrhage [9]. Dutton et al. reported 5 cases involving trauma patients treated with rFVIIa for control of hemorrhage in retrospective case reviews, and suggested that the use of rFVIIa effectively controlled hemorrhage in trauma patients [10]. Mayo et al. described 13 patients with life-threatening hemorrhage who experienced a reduction or cessation of bleeding after rFVIIa infusion [11].
Our study also showed that the number of pRBC and FFP units transfused was reduced after rFVIIa administration in the rFVIIa-treated group; the rFVIIa-treated group required approximately 4-8 fewer units than the rFVIIa-untreated group. There was no statistically significant difference in the number of PLT concentrate units transfused between the 2 groups by the independent samples t test; however, multiple regression analysis revealed that the number of PLT concentrate units transfused was significantly reduced in the rFVIIa-treated patients compared to the rFVIIa-untreated patients (P <0.001).
In order to aid physicians, Goodnough et al. suggested hospital-based transfusion service guideline policies for rFVIIa therapy [12]. Loudon et al. determined that intervention with rFVIIa after transfusion of 14 units of pRBCs is cost effective due to conservation of blood components and a reduction in the duration of ICU stay [13]. Grounds et al. assessed the efficacy and safety of rFVIIa in 45 patients with massive bleeding requiring >14 units of pRBCs. They concluded that the use of rFVIIa reduced blood loss, transfusion requirements, and the mortality rate [14]. Rizoli et al. performed a retrospective cohort study of 38 rFVIIa-treated patients and 204 rFVIIa-untreated patients, and showed that rFVIIa might improve early survival of trauma patients with life-threatening hemorrhage [15].
In our study, we selected patients who received ≥8 units of pRBCs within the first 24 hr of hospitalization, and showed that the use of rFVIIa improved clinical outcomes, reduced the duration of both the ICU and total hospital stays, and was cost effective in massive bleeding patients who required ≥8 units of pRBCs.
Cameron et al. reviewed the Australian and New Zealand Haemostasis Registry databases and reported that rFVIIa decreased or stopped bleeding in 59% of cases for which efficacy was reported. Body temperature, arterial pH, and ISS were shown to contribute to the best model for prediction of mortality [16]. In our study, we recognized that older age, lower initial systolic blood pressure, more acidic arterial pH, higher lactic acid level, prolonged PT and aPTT values, and massive transfusion of pRBCs and FFP were statistically significant prognostic factors of worse survival in multiple trauma patients with life-threatening hemorrhage.
Boffard et al. and Rizoli et al. conducted 2 randomized, placebo-controlled, double-blind clinical trials to evaluate the efficacy and safety of rFVIIa in trauma patients with severe bleeding. The number of pRBCs, FFP, and PLT concentrate units transfused was significantly reduced in the rFVIIa-treated group compared to the placebo group, and the need for massive transfusion was reduced. Moreover, trends toward a reduction in mortality and critical complications were observed [17, 18].
Martinowitz and Michaelson established a Multidisciplinary Task Force and published guidelines for the use of rFVIIa in cases of uncontrolled bleeding. They recommended that an initial dose of 200 µg/kg of rFVIIa, followed by 2 100 µg/kg doses administered 1 and 3 hr after the first dose may reduce RBC transfusion requirements, the need for massive transfusion, and the incidence of respiratory failure in patients with blunt trauma [19]. A European committee also developed consensus guidelines for use of rFVIIa in patients with life-threatening hemorrhage based on a review of MEDLINE, EMBASE, and Cochrane databases [20]. In 2 placebo-controlled pharmacokinetic studies of rFVIIa in trauma patients, the mean clearance of rFVIIa was 40 mL·kg-1·h-1 and the terminal half-life was 2.4 hr. Increased intra- and inter-patient variability occurred in the volume of distribution and clearance of rFVIIa, and this increased variability was mainly related to transfusion requirements, blood loss, and bleeding rate [21].
However, whether or not rFVIIa treatment in patients with multiple trauma improves clinical outcomes and is cost effective is still a controversial issue. Yank et al. evaluated the benefits and harms of rFVIIa use for 5 off-label indications, including trauma, by reviewing and analyzing 10 databases. They concluded that there was no difference in mortality from trauma; however, the risk for thromboembolism did not increase, but the risk for acute respiratory distress syndrome decreased [22]. Lin et al. searched several databases up to 2009 and also concluded that the effectiveness of rFVIIa remains unproven [23].
Joo et al. [8] first reported the off-label use of rFVIIa in 15 multiple trauma patients in Korea. They compared the units of pRBCs and FFP transfused per hr before and 6 hr after rFVIIa administration, vital signs (systolic blood pressure and heart rate) before and after rFVIIa administration, and changes in PT and aPTT before and 30, 60, and 180 min after rFVIIa administration. They concluded that the units of pRBCs and FFP transfused per hour were significantly reduced and PT and aPTT were significantly corrected with no difference in vital signs. In our study, the PT and aPTT of the rFVIIa-treated group were corrected until 6 hr after rFVIIa administration.
There were significant differences between the study by Joo et al. [8] and our study; we selected 18 rFVIIa-treated patients (including 12 patients in the study by Joo et al.) and 36 rFVIIa-untreated patients from all multiple trauma patients using our own inclusion and exclusion criteria; we compared the total transfused units (including PLT concentrate) within the first 24 hr of admission and during the entire hospital stay between the 2 groups; we also analyzed the clinical outcomes (including the duration of the ICU and total hospital stays); we also examined the mortality rate within the first 24 hr and 30 days; Transfusion costs, hospital stay costs, and total treatment costs were calculated and compared between the 2 groups.
The major adverse effects of rFVIIa, including thromboembolic events such as acute myocardial infarction or cerebrovascular infarction, did not occur in the rFVIIa-treated patients or the rFVIIa-untreated patients.
Treatment with rFVIIa could significantly reduce transfusion costs and hospital stay costs; however, the total treatment costs, including the price of the rFVIIa drug, were not cost-effective. However, we presume that in the future the total treatment cost will be cost-effective if the Food and Drug Administration (FDA) approves the use of rFVIIa for trauma patients and the price of rFVIIa drug is reduced.
In conclusion, the number of units transfused was significantly reduced after rFVIIa administration within the first 24 hr of admission in the rFVIIa-treated patients, while the number of units transfused was not significantly reduced in the rFVIIa-untreated patients. The rFVIIa-treated patients required approximately 4-8 fewer units of pRBCs and FFP than the rFVIIa-untreated patients. The number of PLT concentrate units transfused did not differ significantly between the 2 groups by the independent samples t test; however, the number of PLT concentrate units transfused was significantly reduced in the rFVIIa-treated patients compared to the rFVIIa-untreated patients by multiple regression analysis. Treatment with rFVIIa led to significantly better clinical outcomes and a reduced 24-hr mortality rate without a reduction in the 30-day mortality rate. Treatment with rFVIIa was cost-effective for transfusion costs and hospital stay costs; however, the total treatment costs, including the price of the rFVIIa drug, were not cost-effective. Therefore, our study showed that the use of rFVIIa could be helpful as a supplementary therapy to improve clinical outcomes, reduce the 24-hr mortality rate, reduce transfusion costs and hospital stay costs, and decrease blood transfusion requirements in multiple trauma patients with life-threatening hemorrhage.
Figures and Tables
Fig. 1
Transfusion volume (units) of pRBCs (P<0.0001), FFP (P<0.0001), and PLT concentrate (P=0.0085) before (pre) and after (post) rFVIIa administration within the first 24 hr of hospital admission in the 18 rFVIIa-treated patients. Data are presented as box plots with median lines.
Abbreviations: RBC, red blood cells; FFP, fresh frozen plasma; PLT, platelet concentrates; rFVIIa, recombinant activated factor VII.

Fig. 2
Changes in mean (A) hemoglobin, (B) platelet count, (C) PT, and (D) aPTT after rFVIIa administration in the rFVIIa-treated group (N=18, straight lines) and within the first 24 hr of admission in the rFVIIa-untreated group (N=36, dotted lines).
Abbreviations: PT, prothrombin time; aPTT, activated partial thromboplastin time; rFVIIa, recombinant activated factor VII.

Table 1
Demographics and initial laboratory results of the rFVIIa-treated and rFVIIa-untreated groups

Table 4
Characteristics, initial vital signs, and initial laboratory results at admission, and units transfused for rFVIIa-treated patients who were alive vs. dead 30 days after admission
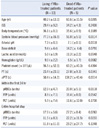
The Mann-Whitney U test was used to calculate P values. The data shown are the mean (±SD).
Abbreviations: rFVIIa, recombinant activated factor VII; ISS, injury severity score; PT, prothrombin time; aPTT, activated partial thromboplastin time; pRBCs, packed red blood cells; FFP, fresh frozen plasma; PLT, platelet concentrates.
References
2. Rudolph R, Boyd CR. Massive transfusion: complications and their management. South Med J. 1990. 83:1065–1070.
3. Pike AC, Brzozowski AM, Roberts SM, Olsen OH, Persson E. Structure of human factor VIIa and its implications for the triggering of blood coagulation. Proc Natl Acad Sci. 1999. 96:8925–8930.

4. Ingerslev J. Efficacy and safety of recombinant factor VIIa in the prophylaxis of bleeding in various surgical procedures in hemophilic patients with factor VIII and factor IX inhibitors. Semin Thromb Hemost. 2000. 26:425–432.

5. Franchini M. Recombinant factor VIIa: a review on its clinical use. Int J Hematol. 2006. 83:126–138.

6. Kenet G, Walden R, Eldad A, Martinowitz U. Treatment of traumatic bleeding with recombinant factor VIIa. Lancet. 1999. 354:1879.

7. Goodnough LT, Shander AS. Recombinant factor VIIa: safety and efficacy. Curr Opin Hematol. 2007. 14:504–509.

8. Joo YM, Yeom SR, Ryu JH, Jeong JW, Kim YI, Min MK, et al. Successful hemostasis by the use of recombinant factor VIIa in uncontrolled active hemorrhage of multiple trauma patients. J Korean Soc Emerg Med. 2011. 22:22–29.
9. Martinowitz U, Kenet G, Segal E, Luboshitz J, Lubetsky A, Ingerslev J, et al. Recombinant activated factor VII for adjunctive hemorrhage control in trauma. J Trauma. 2001. 51:431–438.

10. Dutton RP, Hess JR, Scalea TM. Recombinant factor VIIa for control of hemorrhage: early experience in critically ill trauma patients. J Clin Anesth. 2003. 15:184–188.

11. Mayo A, Misgav M, Kluger Y, Greenberg R, Pauzner D, Klausner J, et al. Recombinant activated factor VII (NovoSeven) : addition to replacement therapy in acute, uncontrolled and life-threatening bleeding. Vox Sang. 2004. 87:34–40.
12. Goodnough LT, Lublin DM, Zhang L, Despotis G, Eby C. Transfusion medicine service policies for recombinant factor VIIa administration. Transfusion. 2004. 44:1325–1331.

13. Loudon B, Smith MP. Recombinant factor VIIa as an adjunctive therapy for patients requiring large volume transfusion: a pharmacoeconomic evaluation. Intern Med J. 2005. 35:463–467.

14. Grounds RM, Seebach C, Knothe C, Paluszkiewicz P, Smith TS, Kasal E, et al. Use of recombinant activated factor VII (Novoseven) in trauma and surgery: analysis of outcomes reported to an international registry. J Intensive Care Med. 2006. 21:27–39.

15. Rizoli SB, Nascimento B Jr, Osman F, Netto FS, Kiss A, Callum J, et al. Recombinant activated coagulation factor VII and bleeding trauma patients. J Trauma. 2006. 61:1419–1425.

16. Cameron P, Philips L, Balogh Z, Joseph A, Pearce A, Parr M, et al. The use of recombinant activated factor VII in trauma patients: Experience from the Australian and New Zealand haemostasis registry. Injury. 2007. 38:1030–1038.

17. Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, et al. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma. 2005. 59:8–15.

18. Rizoli SB, Boffard KD, Riou B, Warren B, Lau P, Kluger Y, et al. Recombinant activated factor VII as an adjunctive therapy for bleeding control in severe trauma patients with coagulopathy: subgroup analysis from two randomized trials. Crit Care. 2006. 10:R178.
19. Martinowitz U, Michaelson M. Guidelines for the use of recombinant activated factor VII (rFVIIa) in uncontrolled bleeding: a report by the Israeli multidisciplinary rFVIIa task force. J Thromb Haemost. 2005. 3:640–648.

20. Vincent JL, Rossaint R, Riou B, Ozier Y, Zideman D, Spahn DR. Recommendations on the use of recombinant activated factor VII as an adjunctive treatment for massive bleeding - a European perspective. Crit Care. 2006. 10:R120.
21. Klitgaard T, Tabanera y Palacios R, Boffard KD, Iau PT, Warren B, Rizoli S, et al. Pharmacokinetics of recombinant activated factor VII in trauma patients with severe bleeding. Crit Care. 2006. 10:R104.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download