Introduction
Gestational trophoblastic disease (GTD) defines a heterogeneous group of lesions arising from abnormal cellular proliferation of placental villous trophoblast. Subtypes of GTD include partial and complete hydatidiform mole and gestational trophoblastic neoplasia (GTN). GTNs can be pathologically classified into invasive mole, choriocarcinoma, placental site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor (ETT) with the potential for local invasion and metastasis, leading to fatal condition if left untreated.
PSTT is extremely rare tumor type in this group, originates from intermediate trophoblastic cells in implantation site. It consists 0.25–5% of all gestational trophoblastic tumors with an estimated incidence of 1 in 100,000 pregnancies [
1]. This disease was first recognized in 1976, Kurman et al. [
2] used the term ‘trophoblastic pseudotumor,’ established the trophoblastic origin of the lesion and it was thought at first to be a benign variant of gestational trophoblastic tumors. After reports of several fatal cases, PSTT was named to describe the malignant potential of this tumor in 1981 [
3]. Since this initial description, approximately 300 cases have been reported in the literature [
4]. Most PSTTs behave in a benign feature, but approximately 10–15% of patients are clinically malignant and the mortality rate is 25% in reported literature [
5].
PSTT is distinct from other type of GTN, characterized by slow growth, low concentration of serum beta human chorionic gonadotropin (β-hCG), the late onset metastatic potential, resistance to chemotherapy. The treatment of PSTT is controversial but hysterectomy is an important and valuable primary treatment, in particular for the disease localized to the uterus [
6]. In this study, we showed clinical characteristics and outcomes of 6 patients of PSTT treated successfully by hysterectomy alone in our hospital in addition to one case presentation.
Results
We identified 418 registrations for GTD and 6 patients had histologically confirmed PSTT for an overall frequency of 1.4%, reflecting the rarity of this condition relative to other forms of GTD. The results of features, treatment, and outcome for all patients are summarized in
Table 1.
Table 1
Clinical features, treatment, outcome in 6 patients with placental site trophoblastic tumor

|
Characteristic |
Patient number |
|
1 |
2 |
3 |
4 |
5 |
6 |
|
Age (yr) |
29 |
32 |
32 |
29 |
31 |
33 |
|
AP |
N/A |
Term |
Term |
Term |
Term |
Term |
|
Interval from AP (mon) |
N/A |
6 |
N/A |
3 |
10 |
12 |
|
Presenting features |
IVB |
IVB |
IVB |
IVB |
IVB |
Amenorrhea |
|
β-hCG at diagnosis (mIU/mL) |
171.0 |
32.0 |
0.4 |
35.9 |
2.6 |
903.5 |
|
FIGO stage |
I |
I |
I |
I |
I |
I |
|
Mets (No./site) |
- |
- |
- |
- |
- |
- |
|
Myometrial invasion |
Superficial |
Deep |
Superficial |
Superficial |
N/A |
Deep |
|
Mitotic index, (mitoses/0 HPF) |
N/A |
N/A |
N/A |
8 |
N/A |
4 |
|
IHC |
N/A |
N/A |
Ki-67 (5–10%), CK 8 & 18, CD 10 |
Ki-67 (>10%) |
Ki-67 (>10%), hCG |
Ki-67 (20%), hPL, hCG, CK 7 |
|
Dx procedure |
D/E |
D/C/B |
D/C/B |
D/C/B |
D/C/B |
Image |
|
Surgery |
TAH |
TAH, RSO, LS, PLND |
SPA-H, BS |
TAH, ROC |
LAVH |
TAH, BS |
|
Chemotherapy |
- |
- |
- |
- |
- |
- |
|
Outcome |
NED |
NED |
NED |
NED |
NED |
NED |
|
Follow-up (mon) |
2 |
84 |
46 |
73 |
48 |
1 |
The median age of the patients was 31 years (range 29–33 years). Among 5 patients who had documented antecedent pregnancy prior to the diagnosis, a full-term pregnancy was identified in all 5 cases: In one case (patient 1), there was no documented information about pregnancies prior to the diagnosis of PSTT. In 4 patients who had documented interval between termination of previous pregnancy and diagnosis, the median interval from pregnancy to diagnosis of PSTT was 8 months (range 3–12 months); Information about period between antecedent pregnancy and diagnosis was not available in one patient (patient 3). The serum β-hCG titers exhibited broad range of relatively low concentration at the diagnosis: The median β-hCG was 34.0 mIU/mL (range 0.4–903.5 mIU/mL).
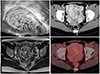
Five of the 6 patients had irregular vaginal bleeding at presentation and underwent endometrial biopsy (dilatation/curettage or dilatation/evacuation) for diagnosis, which provided positive diagnosis of PSTT; patient 6 was tentatively diagnosed as choriocarcinoma or PSTT before surgery by clinical features and MRI findings (
Fig. 1) and biopsy was not performed before surgery due to risk of severe bleeding during the biopsy. There was no definite evidence of metastasis in the radiologic studies in all 6 patients, so they underwent hysterectomy.
Fig. 1
Image findings of case 6. (A) Transvaginal ultrasonography showed a multicystic mass measuring 7.8×5.1 cm in the posterior uterine wall with abundant blood flow, suspected gestational trophoblastic neoplasia (GTN). (B) Abdomen-pelvis computed tomography. (C) Pelvis magnetic resonance imaging showed about 7.2 cm sized mass with internal vascular structure in uterus. (D) Positron emission tomography-computed tomography.
The pathologic reports of 6 patients were reviewed and confirmed as PSTT. All tumors were characterized microscopically by nuclear and cytoplasmic pleomorphism of intermediate trophoblast cells. Interestingly, all 6 cases presented with disease confined to the uterus (
Fig. 2). Deep myometrial invasion (tumor infiltration of more than one half of the myometrium) was observed in 3 (50%) patients and superficial invasion (tumor infiltration less than one half of myometrium) in 3 (50%) patients.
Fig. 2
(A) Macroscopic features of placental site trophoblastic tumor (PSTT): about 6.5×4.2 cm sized irregular mass with infiltration of full depth of myometrium. (B) The PSTT had monomorphic intermediate trophoblastic cells extensively infiltrating the myometrium. (C) The tumor cells are large, mononucleate placental site intermediate trophoblast with scattered multinucleated cells. The cells have abundant eosinophilic cytoplasm and marked nuclear pleomorphism with large convoluted nuclei (hematoxylin and eosin staining, original magnification ×50, ×50).

In 4 of 6 cases, results of immunohistochemistry were available. The tumor cells were labeled with Ki-67 staining ranged from 5–20%. The tumor cells stained positive for hPL in 2 cases (patient 5, 6) tested for hPL (
Fig. 3). Immunoreactivity for hCG was seen in 2 cases (patient 5, 6) tested for hCG but was focally positive in both cases.
Fig. 3
Immunohistochemical findings of case 6. (A) Tumor cells were positive for human placental lactogen (hPL). (B) Tumor cells were focally positive for human chorionic gonadotropin (hCG). (C) About 20% of the tumor cells were positive for Ki-67. (D) Tumor cells were strongly positive for cytokeratin 7 (CK 7).

After surgical treatment, chemotherapy was not given since all 6 patients were stage I. The median follow-up was 47 months (range 2–84) and none of them have evidence of disease recurrence in the follow-up period.
Discussion
PSTT usually presents with irregular vaginal bleeding months or even years after the last pregnancy. Other presentations are amenorrhea, palpable abdominal mass and postmenopausal bleeding in decreasing order of frequency. Rare symptoms are contingent on site of metastasis such as cough, dizziness, headache, or back pain. In this study, we also observed irregular vaginal bleeding was the most common symptom (83.3%,
Table 1).
In the normal implantation site, the invasion of intermediate trophoblast is tightly regulated and is confined to the inner third of the myometrium. In contrast, the tumor cells of PSTT are highly invasive and infiltrate deeply into the myometrium, occasionally penetrating through the uterine wall. One half (50%) of our patients exhibited deep myometrial invasion (
Table 1). In pathologic finding, PSTT shows deep infiltration into the myometrium and the tumor cells are large, mononucleate placental site intermediate trophoblasts with scattered multinucleated cells. The cells have abundant eosinophilic cytoplasm and marked nuclear pleomorphism with large convoluted nuclei. These findings were consistent with our cases (
Fig. 2B and C)
Clinically, distinguishing PSTT from other types of GTN or other neoplastic disease is important as far as treatment plans are concerned. Immunohistochemistry examination has distinct value in diagnosing PSTT and is currently considered to be the golden standard for diagnosis of PSTT [
8]. The tumor cells usually have a high level of Ki-67 expression which is a marker of cell proliferation activity in tissues [
9]. Determining the Ki-67 labeling index can be useful for the differential diagnosis of trophoblastic tumors. Choriocarcinomas are diffusely labeled with Ki-67 (>50%), whereas most of PSTT contain approximately 8–20% of Ki-67 positive cells [
9]. In our cases, we found Ki-67 expression in 5–20% consistent with literature (
Table 1,
Fig. 3). hPL, normally secreted by syncytiotrophoblast, is highly expressed in the trophoblasts of PSTT and is expressed in most of the trophoblastic cells of PSTT. hCG and placental alkaline phosphatase are secreted by syncytiotrophoblast cells, these markers are rarely evident or even absent in a PSTT [
9]. In this study, we also observed diffuse hPL expression and relatively focal expression of hCG in patient 5, 6 specimens (
Fig. 3).
Histological findings from a PSTT are specific, but it is difficult to distinguish the benign or malignant characteristics of a PSTT. A high mitotic rate of more than 5/10 high-power field and substantial hemorrhaging and necrosis within the tumor has been suggested malignancy and the essential pathologic predictor for disease recurrence [
10].
Hysterectomy is recommended for patients with PSTT if fertility need not be preserved and the disease is localized to the uterus. Additionally, oophorectomy is not considered routinely because ovarian metastases are uncommon and an oophorectomy cannot improve prognosis [
11]. In a recent study, PSTT tend to spread through lymphatic pathways, resulting in relative resistance to chemotherapy [
12]. But the role of lymphadenectomy in the surgical management of PSTT is still controversial [
13]. In the current study, all our 6 patients were treated successfully by hysterectomy alone since they were stage I and alive without evidence of disease until last follow-up indicating surgery is appropriate treatment for localized PSTT. Although there is no conclusive evidence to support adjuvant chemotherapy in PSTT, it is reasonable to consider adjuvant chemotherapy in patients with risk factors associated with bad prognosis. In the literature [
14], adjuvant chemotherapy may be considered for patients with a time interval of (≥4 years) from the antecedent pregnancy and advanced stage (FIGO stage III–IV) disease. While some investigators suggest adjuvant chemotherapy in stage I disease with deep myometrial invasion or serosal involvement, there is currently no data to support this recommendation [
13]. Although there are several chemotherapy regimens used to treat PSTT with metastatic disease, optimal regimen is not identified because of the rarity of disease and the lack of controlled trials. Despite the lack of any conclusive differences between regimens, etoposide, methotrexate, actinomycin D/ cyclophosphamide, vincristine [
15] or etoposide, methotrexate, actinomycin D/ etoposide, cisplatin [
16] are the preferential adjuvant chemotherapy (
Table 2).
Table 2
Summary of reports of placental site trophoblastic tumor (PSTT) in literature

|
Author |
No. of cases |
Age (mean, yr) |
AP |
Interval to AP (median, mon) |
β-hCG (median, IU/L) |
Prognostic risk factors |
Treatment (percent of patients) |
Outcome (survival or NEDa)) |
|
Chang et al. [20] |
88 |
30.0 |
Term/preterm (59%) |
12.00 |
467.3 |
FIGO stage |
Hysterectomy (84%) |
OS |
|
Abortion (26%) |
Adjuvant CTx (25%) |
- Stage I/II: 93.5% |
|
H-mole (13%) |
Primary CTx (34%) |
- Stage III/IV: 33.3% |
|
Choriocarcinoma (2%) |
|
|
|
Unknown (13%) |
|
|
|
Feltmate et al. [15] |
13 |
31.2 |
Term (54%) |
16.50 |
257.0 |
Interval from AP (>24 mon) |
Hysterectomy (92%) |
Not reported |
|
Abortion (38%) |
Mitotic index |
Adjuvant CTx (55%) |
|
H-mole (8%) |
Metastatic disease |
Primary CTx (44%) |
|
|
- Immediate CTx (≤1 wk from surgery) in high risk |
|
|
- EMA-EP for adjuvant CTx |
|
Hassadia et al. [17] |
17 |
36.1 |
Term (59%) |
18.00 |
13,923 |
FIGO stage (IV) |
Hysterectomy (65%) |
NED |
|
Abortion (12%) |
Interval from AP (>48 mon) |
Adjuvant CTx (29%) |
- Stage I: 47% |
|
Unknown (29%) |
β-hCG (>10,000) |
Primary CTx (35%) |
- Stage III: 29% |
|
Age (>40 yr) |
|
|
|
Baergen et al. [6] |
55 |
32.0 |
Term (57%) |
18.00 |
691.0 |
FIGO stage (III/IV) |
Hysterectomy (89%) |
OS: 80–86% |
|
Abortion (17%) |
Interval from AP (>24 mon) |
Adjuvant CTx (35%) |
- Stage I: 91–92% |
|
H-mole (26%) |
β-hCG |
|
- Stage III/IV: 41% |
|
Deep invasion |
|
|
|
Mitotic index |
|
|
|
Clear cytoplasm |
|
|
|
Tumor necrosis |
|
|
|
Age (>35 yr) |
|
|
|
Schmid et al. [18] |
62 |
34.6 |
Term (60%) |
18.00 |
<1,000.0 |
FIGO stage |
Hysterectomy (85%) |
OS: 70% |
|
Stillbirth (16%) |
Interval from AP (>48 mon) |
Adjuvant CTx (13%) |
- Stage I: 93% |
|
Abortion (10%) |
Prognostic score |
Primary CTx (32%) |
- Stage II: 52% |
|
H-mole (15%) |
Mitotic index |
|
- Stage III/IV: 49% |
|
β-hCG |
|
|
|
Age |
|
|
|
No. of metastasis |
|
|
|
Lan et al. [12] |
5 |
36.4 |
Term (60%) |
7.00 |
50.0 |
LN metastasis |
Hysterectomy (100%) |
NED |
|
Stillbirth (20%) |
Adjuvant CTx (60%) |
- Stage I: 40% |
|
Abortion (20%) |
|
- Stage II: 20% |
|
Chen et al. [16] |
17 |
31.7 |
Term (88.2%) |
14.20 |
556.7 |
Not reported |
Hysterectomy (100%) |
NED |
|
Abortion (12.8%) |
Adjuvant CTx (47%) |
- Stage I: 82.3% |
|
- EMA-CO for adjuvant CTx |
- Stage III:12.8% |
|
Hyman et al. [21] |
17 |
34.1 |
Term (53%) |
9.00 |
132.0 |
FIGO stage |
Hysterectomy (94.1%) |
NED |
|
Abortion (35%) |
Interval from AP (>12 mon) |
Adjuvant CTx (100%) |
- Stage I: 85.7% |
|
H-mole (12%) |
Term AP |
- Beneficial Tx for Stage I: hysterectomy only |
- Stage III/IV: 33% |
|
β-hCG |
|
|
|
Age (>40 yr) |
|
|
|
Zhao et al. [19] |
108 |
31.8 |
Term/preterm (62%) |
25.13 |
154.3 |
FIGO stage |
Hysterectomy (78.7%) |
OS: 93.5% |
|
Abortion (28.7%) |
Interval from AP (>36 mon) |
Preserve fertility Txb) (21.3%) |
- Stage I: 100% |
|
H-mole (7.4%) |
Prognostic score |
- No significant difference in stage I PSTT |
- Stage III/IV: 87.9% |
|
Unknown (1.9%) |
Tumor necrosis |
Adjuvant CTx (79.6%) |
|
|
Deep invasion (>50%) |
|
|
|
Our study |
6 |
31.0 |
Term (83.3%) |
8.00 |
34.0 |
Not determined |
Hysterectomy (100%) |
NED |
|
Unknown (16.7%) |
- Stage I: 100% |
In 2002, the FIGO defined criteria for the diagnosis of postmolar disease and adopted a combined anatomic staging and modified World Health Organization risk factor scoring system for GTN [
7]. The factors are FIGO staging, age, type and interval of antecedent pregnancy, β-hCG at diagnosis, tumor size, site and number of metastasis, previous chemotherapy. But, individual FIGO risk scores were not a reliable predictor and do not correlate well with outcome for most patients of PSTT since the range was narrow and clustered around the low/high risk cut off [
17]. So, PSTT is classified separately and it is now recommended that scores should not be used to guide treatment in PSTT since chemotherapy is not routinely given in patients with PSTT [
14].
There is still controversy for potential prognostic factors in PSTT. In the literature, several risk factors appear to be associated with clinical outcomes and
Table 2 summarized the reported analyses. These are FIGO stage, advanced age, an interval from the preceding pregnancy, deep myometrial invasion, a high mitotic rate and high β-hCG level; further studies are warranted to determine accurate significance of each risk factors.
The survival rate has been known approximately 100% for non-metastatic disease and 50–60% for metastatic disease [
14]. According to the retrospective study [
18], long term survival for stage I patients with PSTT with low risk disease after hysterectomy is nearly 90% at 10 years. In patients with stage II–IV disease, surgical resection and chemotherapy have resulted in approximately 50% overall survival at 10 years. In patients with stage I disease who underwent hysterectomy for initial treatment have excellent prognosis (overall survival range 92–100%), except one study [
12] with small number of cases (
Table 2). If young patients want to preserve fertility, they should be carefully counseled. Zhao et al. [
19] demonstrated that fertility preservation is feasible for young patients with localized PSTT lesions with the intention of preserving fertility. They reported that the patients had preserved fertility neither resulted in poor outcome nor an increased risk of relapse. However, the preservation of fertility is not recommended routinely, especially for patients whose lesion is diffuse or who have adverse prognostic factors.
In conclusion, PSTT is the extremely rare type of GTN with unpredictable biological behavior. The diagnosis should be suspected when intrauterine mass is observed in sonographic and/or radiologic examinations and the serum β-hCG titer is elevated in low level in patients presented irregular vaginal bleeding with varying interval after antecedent term pregnancy. Pathologic and immunohistochemistry findings are most important for the accurate diagnosis. Total hysterectomy remains main stay for the primary treatment of patients with PSTT, in particular disease confined to uterus. In this study, we also demonstrated hysterectomy alone was successful for the treatment of stage I disease of PSTT. Although PSTT has been known relatively resistant to chemotherapy, further studies are necessary to determine the role of chemotherapy in this disease as well as appropriate indication of chemotherapy in stage I disease.









 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download