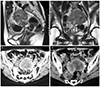
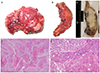
1. Pins MR, Young RH, Daly WJ, Scully RE. Primary squamous cell carcinoma of the ovary. Report of 37 cases. Am J Surg Pathol. 1996; 20:823–833.
2. Stamp GW, McConnell EM. Malignancy arising in cystic ovarian teratomas. A report of 24 cases. Br J Obstet Gynaecol. 1983; 90:671–675.
3. Rekhi B, Parikh P, Deodhar KK, Menon S, Maheshwari A, Kerkar R, et al. Squamous carcinoma coexistent with teratoma of ovary: a clinicopathological study of 12 cases diagnosed over a 10-year period at a tertiary cancer referral center. J Cancer Res Ther. 2015; 11:211–215.
4. Kikkawa F, Nawa A, Tamakoshi K, Ishikawa H, Kuzuya K, Suganuma N, et al. Diagnosis of squamous cell carcinoma arising from mature cystic teratoma of the ovary. Cancer. 1998; 82:2249–2255.
5. Kobayashi H, Sumimoto K, Moniwa N, Imai M, Takakura K, Kuromaki T, et al. Risk of developing ovarian cancer among women with ovarian endometrioma: a cohort study in Shizuoka, Japan. Int J Gynecol Cancer. 2007; 17:37–43.
6. Yamakawa Y, Ushijima M, Kato K. Primary squamous cell carcinoma associated with ovarian endometriosis: a case report and literature review. Int J Clin Oncol. 2011; 16:709–713.
7. Eltabbakh GH, Hempling RE, Recio FO, O'Neill CP. Remarkable response of primary squamous cell carcinoma of the ovary to paclitaxel and cisplatin. Obstet Gynecol. 1998; 91:844–846.
8. Ohtani K, Sakamoto H, Masaoka N, Shimada K, Kanaeda T, Kurihara M, et al. A case of rapidly growing ovarian squamous cell carcinoma successfully controlled by weekly paclitaxel-carboplatin administration. Gynecol Oncol. 2000; 79:515–518.
9. Park JW, Bae JW. Pure primary ovarian squamous cell carcinoma: a case report and review of the literature. Oncol Lett. 2015; 9:321–323.
10. Maruyama T, Sakai K. A case report of pure primary squamous cell carcinoma of the ovary. Jpn J Gynecol Oncol. 2009; 27:69–75.
11. Nakamura Y, Kamei T, Shinagawa M, Sakamoto Y, Miwa I. Case of pure ovarian squamous cell carcinoma resistant to combination chemotherapy with paclitaxel and carboplatin but responsive to monotherapy with weekly irinotecan. J Obstet Gynaecol Res. 2015; 41:809–812.
12. Park JY, Song JS, Choi G, Kim JH, Nam JH. Pure primary squamous cell carcinoma of the ovary: a report of two cases and review of the literature. Int J Gynecol Pathol. 2010; 29:328–334.
13. Mai KT, Yazdi HM, Bertrand MA, LeSaux N, Cathcart LL. Bilateral primary ovarian squamous cell carcinoma associated with human papilloma virus infection and vulvar and cervical intraepithelial neoplasia. A case report with review of the literature. Am J Surg Pathol. 1996; 20:767–772.
14. Shimamatsu K, Iemura A, Nakashima O, Taguchi J, Inoue M, Higaki K, et al. Squamous cell carcinoma of the ovary--a case report. Kurume Med J. 1994; 41:177–182.
15. Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009; 374:1331–1338.
16. Sugiyama T, Yakushiji M, Nishida T, Ushijima K, Okura N, Kigawa J, et al. Irinotecan (CPT-11) combined with cisplatin in patients with refractory or recurrent ovarian cancer. Cancer Lett. 1998; 128:211–218.